679453
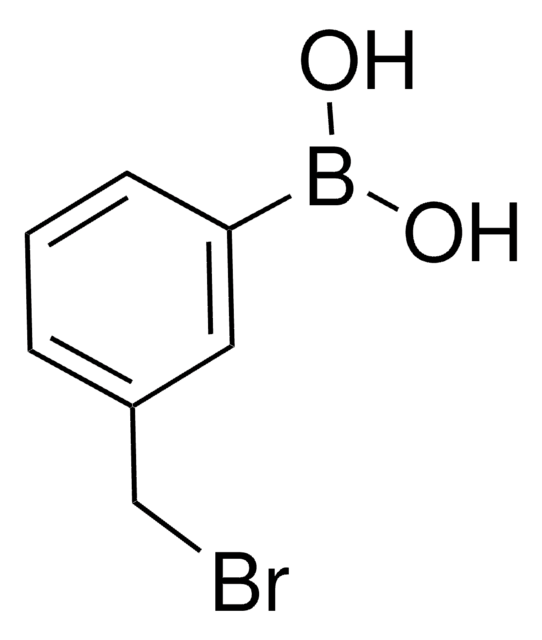
2-(Bromomethyl)phenylboronic acid
Synonym(s):
α-Bromo-o-tolueneboronic acid, o-Boronobenzyl bromide
About This Item
Recommended Products
mp
158-162 °C
Quality Level
storage temp.
2-8°C
SMILES string
OB(O)c1ccccc1CBr
InChI
1S/C7H8BBrO2/c9-5-6-3-1-2-4-7(6)8(10)11/h1-4,10-11H,5H2
InChI key
MYVJCOQGXCONPE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Boronic acid-functionalized benzyl viologen (o-BBV) from 4,4′-bipyridine. o-BBV finds its application as a chemosensor to sense glucose in aqueous water.
- 2-(azidomethyl)phenylboronic acid, which is further employed in the preparation of isoquinoline derivatives.
- Aryl boronic acid derivatives as fluorescent probe for hydrogen peroxide detection.
- Studies of carbon-boron bond cleavage of phenylboronate-pendant cyclen
- Development of a sensory system to detect glucose or other monosaccharides and hydroxycarboxylates
- Synthesis of boronated triaryl and tetraaryl phosphonium salts used in cytotoxicity studies
- Colorimetry and fluorometry mercury determination with chemosensors composed of rhodamine and boronic acid groups
- Synthesis of imidazolium-containing boronic acids used as fluoride ion sensors
- Reactions with adenine for molecules with antiinflammatory antitumor activities
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service