All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8O3
CAS Number:
Molecular Weight:
128.13
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
59-62 °C (lit.)
functional group
carboxylic acid
ketone
SMILES string
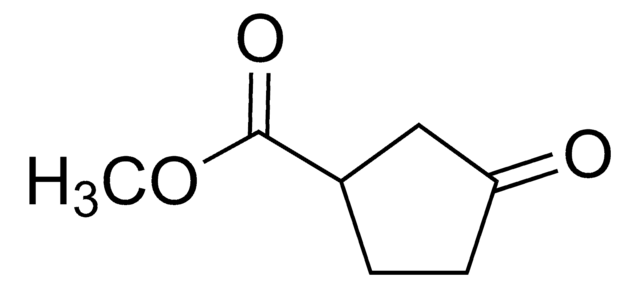
OC(=O)C1CCC(=O)C1
InChI
1S/C6H8O3/c7-5-2-1-4(3-5)6(8)9/h4H,1-3H2,(H,8,9)
InChI key
RDSNBKRWKBMPOP-UHFFFAOYSA-N
Related Categories
General description
3-Oxo-1-cyclopentanecarboxylic acid , also known as 3-oxocyclopentanecarboxylic acid, is a keto acid derivative. It undergoes Curtius rearrangement with diphenyl phosphoryl azide and triethylamine in tert-butanol to form the corresponding boc-protected 1-(3-oxo)urea derivative.
Application
3-Oxo-1-cyclopentanecarboxylic acid may be used in the preparation of 3-hydroxycyclopentanecarboxylic acid via hydrogenation.
Substrate used in a study of biohydroxylation with mutants of cytochrome P450 BM-3.
Legal Information
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Studies of Configuration. V. The Preparation and Configuration of cis-3-Methoxycyclopentanecarboxylic Acid.
Noyce D and Fessenden J.
The Journal of Organic Chemistry, 24(5), 715-717 (1959)
Dieter F Münzer et al.
Chemical communications (Cambridge, England), (20), 2597-2599 (2005-05-19)
Substrate engineered, achiral carboxylic acid derivative was biohydroxylated with various mutants of cytochrome P450 BM-3 to give two out of the four possible diastereoisomers in high de and ee. The BM-3 mutants exhibit up to 9200 total turnovers for hydroxylation
Boc-protected 1-(3-oxocycloalkyl) ureas via a one-step Curtius rearrangement: mechanism and scope.
Sun X, et al.
Tetrahedron Letters, 55(4), 842-844 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service