All Photos(1)
About This Item
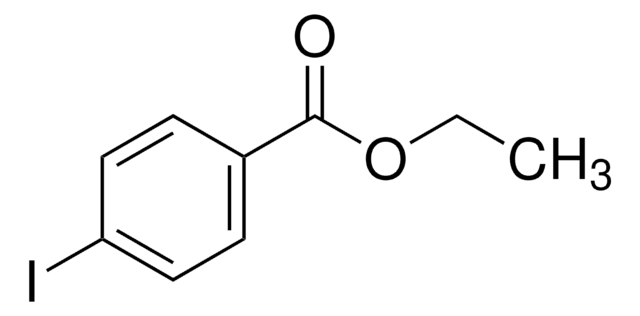
Linear Formula:
IC6H4CO2C2H5
CAS Number:
Molecular Weight:
276.07
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.581 (lit.)
bp
272 °C (lit.)
density
1.64 g/mL at 25 °C (lit.)
functional group
ester
iodo
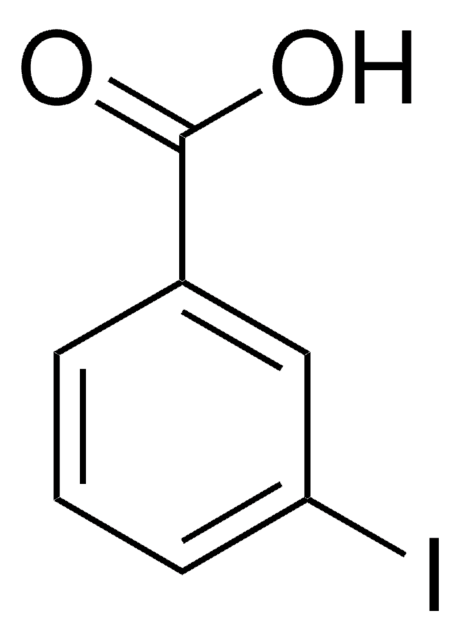
SMILES string
CCOC(=O)c1cccc(I)c1
InChI
1S/C9H9IO2/c1-2-12-9(11)7-4-3-5-8(10)6-7/h3-6H,2H2,1H3
InChI key
POGCXCWRMMXDAQ-UHFFFAOYSA-N
General description
Ethyl 3-iodobenzoate is a halogenated aromatic ester. It affords arylzinc bromide via reaction with i-PrMgBr in THF, followed by reaction with ZnBr2.
Application
Ethyl 3-iodobenzoate may be used to synthesize:
- arylzinc bromide
- functionalized arylmagnesium compound
- ethyl3-phenylbenzoate
- ethyl 3-[(12-tert-butyldimethylsilyloxymethyl-1,12-dicarba-closo-dodecaboran)-1-yl]benzoate
- ethyl 3-(4-methoxy-1-methyl-2-oxo-1,2-dihydroquinolin-3-yl)benzoate
- ethyl 3-(1-methyl-2-oxo-4-phenyl-1,2-dihydroquinolin-3-yl)benzoate
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Copper catalyzed conjugate addition of highly functionalized arylmagnesium compounds to enones.
Varchi G, et al.
Tetrahedron, 56(18), 2727-2731 (2000)
Ni (II)-catalyzed cross-coupling between polyfunctional arylzinc derivatives and primary alkyl iodides.
Giovannini R and Knochel P.
Journal of the American Chemical Society, 120(43), 11186-11187 (1998)
Shinya Fujii et al.
Bioorganic & medicinal chemistry, 17(1), 344-350 (2008-11-22)
A novel series of androgen receptor (AR) ligands bearing an acidic heterocycle with hydrogen-bonding ability as the terminal polar group was developed. Since most non-steroidal AR ligands so far known are structurally limited to nitro- or cyanobenzanilide as the polar
Synthesis of 3, 4-Disubstituted Quinolin-2-(1H)-ones via Palladium-Catalyzed Decarboxylative Arylation Reactions.
Carrer A, et al.
Advanced Synthesis & Catalysis, 355(10), 2044-2054 (2013)
Nonpeptide Arginine Vasopressin Antagonists for Both V1A and V2 Receptors: Synthesis and Pharmacological Properties of 2-Phenyl-4'-((2, 3, 4, 5-tetrahydro-1H-1-benzazepin-1-yl) carbonyl) benzanilide Derivatives.
Matsuhisa A, et al.
Chemical & Pharmaceutical Bulletin, 45(11), 1870-1874 (1997)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service