All Photos(1)
About This Item
Empirical Formula (Hill Notation):
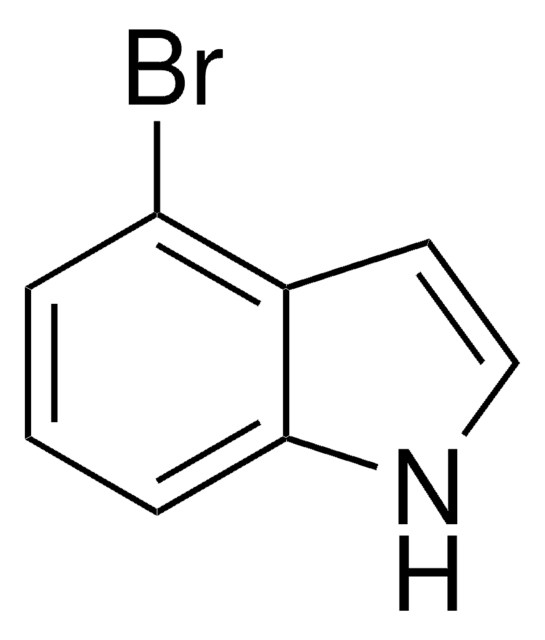
C8H6BrN
CAS Number:
Molecular Weight:
196.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
92-96 °C (lit.)
SMILES string
Brc1ccc2cc[nH]c2c1
InChI
1S/C8H6BrN/c9-7-2-1-6-3-4-10-8(6)5-7/h1-5,10H
InChI key
MAWGHOPSCKCTPA-UHFFFAOYSA-N
General description
6-Bromoindole is an indole derivative. It undergoes palladium-catalyzed reaction with 2-(4-fluorophenyl)ethylpiperazine to afford the carbonylation products.
Application
6-Bromoindole may be used to synthesize:
- 6-alkylthioindole
- 3-acetoxy-6-bromoindole
- 6,6′-dibromoindigo (Tyrian purple)
- 6-acylindoles
- tert-butyl 6-bromoindole-1-carboxylate
Essential starter in 6-substituted indole chemistry.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of N-protected Nortopsentins B and D.
Moody CJ and Roffey JRA.
ARKIVOC (Gainesville, FL, United States), 1, 393-401 (2000)
James R Fuchs et al.
Journal of the American Chemical Society, 126(16), 5068-5069 (2004-04-22)
The first total synthesis of racemic perophoramidine is described. The key step features the highly stereoselective introduction of the vicinial quaternary centers via base-promoted carbon-carbon bond formation between a 3-alkylindole and a 3-bromo-3-alkylindolin-2-one. This transformation presumably proceeds through a conjugate
A facile synthesis of Tyrian purple based on a biosynthetic pathway.
Tanoue Y, et al.
Fisheries Science (Tokyo, Japan), 67(4), 726-729 (2001)
Efficient synthesis of 5-and 6-tributylstannylindoles and their reactivity with acid chlorides in the Stille coupling reaction.
Cherry K, et al.
Tetrahedron Letters, 48(33), 5751-5753 (2007)
Leonardo S Santos et al.
The Journal of organic chemistry, 69(4), 1283-1289 (2004-02-14)
Described are the first enantioselective total syntheses of (+)-arborescidine A ((+)-1), (-)-arborescidine B ((-)-2), and (-)-arborescidine C ((-)-3), via routes that proceeded in five steps and 50% overall yield, eight steps and 61% overall yield, and nine steps and 51%
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service