479403
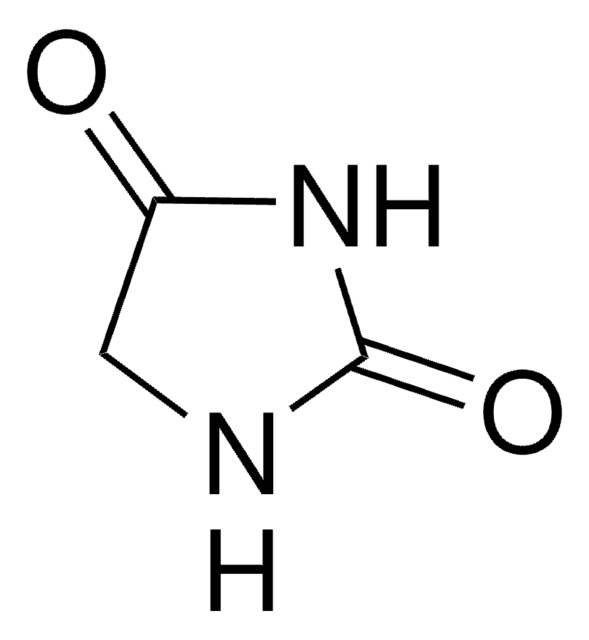
1,5,5-Trimethylhydantoin
98%
Synonym(s):
1,5,5-Trimethyl-2,4-imidazolidinedione, 3,4,4-Trimethyl-2,5-dioxoimidazolidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H10N2O2
CAS Number:
Molecular Weight:
142.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
161-164 °C (lit.)
SMILES string
CN1C(=O)NC(=O)C1(C)C
InChI
1S/C6H10N2O2/c1-6(2)4(9)7-5(10)8(6)3/h1-3H3,(H,7,9,10)
InChI key
ZNYIPTYJBRGSSL-UHFFFAOYSA-N
General description
1,5,5-Trimethylhydantoin (TMH) is a 1,5,5-trisubstituted hydantoin. Its mass spectrum has been recorded and analyzed. The density of TMH is 1.1318g/ml at 25°C.
Application
1,5,5-Trimethylhydantoin (1,5,5-Trimethyl-imidazolidine-2,4-dione) may be used to synthesize 3-bromomethyl-1,5,5-trimethylimidazolid-ine-2,4-dione.
Reactant for:
Z-selective hydroamidation of terminal alkynes with secondary amides and imides
Selective inhibitors of hepatitis C virus NS3 serine protease
Stereoselective addition of imides to alkynes
Reactant for synthesis of:
Selective angiotensin II AT2 receptor agonists with reduced CYP 450 inhibition
N-chlorohydantoins
P2X7 receptor antagonists
Z-selective hydroamidation of terminal alkynes with secondary amides and imides
Selective inhibitors of hepatitis C virus NS3 serine protease
Stereoselective addition of imides to alkynes
Reactant for synthesis of:
Selective angiotensin II AT2 receptor agonists with reduced CYP 450 inhibition
N-chlorohydantoins
P2X7 receptor antagonists
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yaws CL.
Thermophysical Properties of Chemicals and Hydrocarbons, 280-280 (2008)
Mass spectrometric analysis and theoretical calculations of the occurrence of tautomeric structures of hydantoins.
Allegretti PE, et al.
Afinidad, 57(485), 41-49 (2000)
Use of the cascade α-oxo-amidoalkylation/transposition/Π-cationic cyclization of N-acyliminium ions in the synthesis of novel fused heterocyclic N,O-acetals.
Pesquet A, et al.
ARKIVOC (Gainesville, FL, United States), 8, 27-40 (2010)
Zi-Ao Huang et al.
Electrophoresis, 41(3-4), 183-193 (2019-12-19)
In this paper, the development of a simple dilute-and-shoot method for quantifying urinary creatinine by CE-ESI-MS was described. The creatinine analysis time was about 7 min/sample by conventional single injection (SI) method and can be significantly reduced to less than 2 min/sample
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service