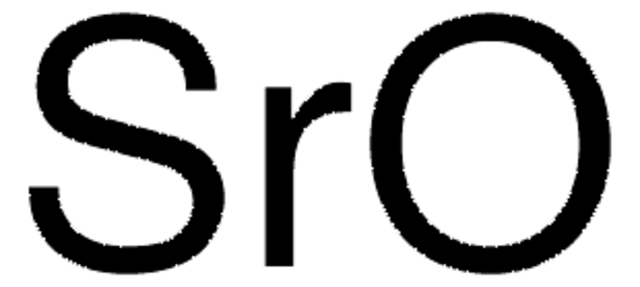289833
Strontium carbonate
≥98%
Synonym(s):
Strontianite
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
SrCO3
CAS Number:
Molecular Weight:
147.63
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
≥98%
form
powder
density
3.7 g/mL at 25 °C (lit.)
SMILES string
[Sr++].[O-]C([O-])=O
InChI
1S/CH2O3.Sr/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2
InChI key
LEDMRZGFZIAGGB-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Strontium carbonate is insoluble in water. It is used predominantly in producing other strontium salts.
Application
Strontium carbonate is used to prepare:
Strontium carbonate is used in pyrotechnics, ceramic ferrites, inexpensive fireworks colorant.
- Barium strontium titanate
- Barium strontium titanate thin films
Strontium carbonate is used in pyrotechnics, ceramic ferrites, inexpensive fireworks colorant.
Storage Class Code
13 - Non Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Photoluminescence properties of europium doped di-strontium magnesium di-silicate phosphor by solid state reaction method
Sahu IP, et al.
Science, 1-6 (2014)
Barium strontium titanate powder obtained by polymeric precursor method
Ries A, et al.
Materials Characterization, 50(2), 217-221 (2003)
Effect of process parameters and post deposition annealing on the optical, structural and microwave dielectric properties of RF magnetron sputtered (Ba 0.5, Sr 0.5) TiO 3 thin films
Saravanan K, et al.
Vacuum, 81(3), 307-316 (2006)
Debabrata Rautaray et al.
Langmuir : the ACS journal of surfaces and colloids, 20(16), 6827-6833 (2004-07-28)
The total biological synthesis of SrCO3 crystals of needlelike morphology arranged into higher order quasi-linear superstructures by challenging microorganisms such as fungi with aqueous Sr2+ ions is described. We term this procedure "total biological synthesis" since the source of carbonate
M Sadeghi et al.
Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine, 67(7-8), 1392-1396 (2009-03-17)
Excitation functions of (86)Y production via (86)Sr(p,xn), (86)Sr(d,xn), (85)Rb(alpha,xn), (85)Rb((3)He,xn), and (nat)Zr(d,alphaxn) reactions were studied by means of ALICE-ASH code and the results were compared with ALICE-91 code and experimental data. The greatest nuclear reaction of cyclotron (86)Y production was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service