All Photos(1)
About This Item
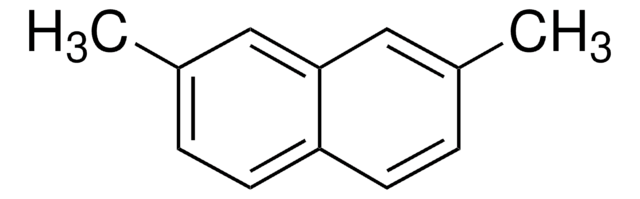
Linear Formula:
C10H6(OCH3)2
CAS Number:
Molecular Weight:
188.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
137-139 °C (lit.)
SMILES string
COc1ccc2ccc(OC)cc2c1
InChI
1S/C12H12O2/c1-13-11-5-3-9-4-6-12(14-2)8-10(9)7-11/h3-8H,1-2H3
InChI key
PPKHAIRFQKFMLE-UHFFFAOYSA-N
Application
2,7-Dimethoxynaphthalene was employed as matrix to investigate the structure of polymetallic porphyrins via matrix-assisted laser desorption/ionization. It was also used in the synthesis of:
- peri-aroylnaphthalene compounds via elective electrophilic aromatic aroylation
- 2-amino-1,2,3,4-tetrahydronaphthalene-6,7-diol
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Concise Synthesis of 2-Amino-1, 2, 3, 4-tetrahydronaphthalene-6, 7-diol ('6, 7-ADTN') from Naphthalene-2, 3-diol.
Goksu S, et al.
Helvetica Chimica Acta, 86(10), 3310-3313 (2003)
Iolinda Aiello et al.
Analytical chemistry, 76(20), 5985-5989 (2004-10-16)
2,7-Dimethoxynaphthalene (DMN) is proposed as matrix to investigate the structure of polymetallic porphyrins through matrix-assisted laser desorption/ionization tandem time-of-flight experiments. The peculiarity of DMN is represented by the formation of molecular radical cations and of some diagnostic fragments only. The
P Prince et al.
Acta crystallographica. Section C, Crystal structure communications, 45 ( Pt 8), 1255-1256 (1989-08-15)
C12H12O2, Mr = 188.2, orthorhombic, P2(1)2(1)2(1), a = 6.109 (3), b = 8.235 (2), c = 19.713 (3) A, V = 991.8 (9) A3, Z = 4, Dx = 1.260 g cm-3, lambda(Mo K alpha) = 0.71073 A, mu =
C L Perrin et al.
Journal of the American Chemical Society, 123(27), 6520-6526 (2001-07-06)
In solution, are the hydrogen bonds in monoprotonated N,N,N',N'-tetramethyl-1,8-naphthalenediamines single- or double-well? To answer this question, isotopic perturbation of equilibrium is applied to a mixture of -d(0), -d(3), -d(6), -d(9), and -d(12) isotopologs. The N-methyls of the 2,7-dimethoxy analogue show
Daichi Hijikata et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 11), o2902-o2903 (2010-01-01)
In the title mol-ecule {systematic name: [2,7-dimethoxy-8-(4-phenoxybenzoyl)naphthalen-1-yl](4-phenoxyphenyl)methan-one}, C(38)H(28)O(6), the 4-phen-oxy-benzoyl units adopt a syn orientation with respect to the naphthalene ring system. The inter-nal benzene rings, A and B, make dihedral angles of 86.72 (5) and 79.22 (5)° with the naphthalene ring
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service