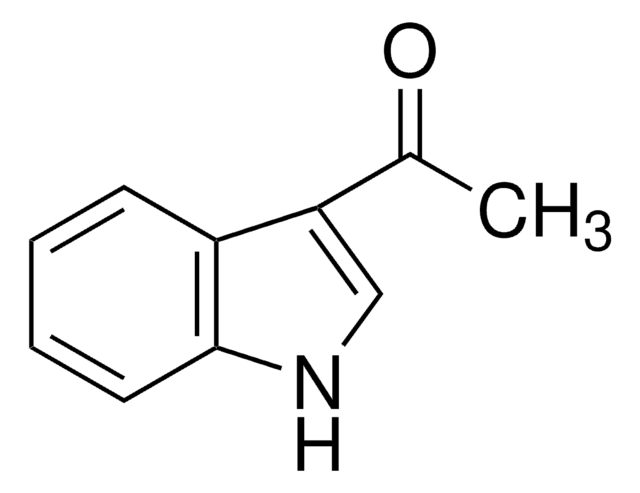
196320
3-Acetylthiophene
98%
Synonym(s):
Methyl-3-thienyl ketone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6OS
CAS Number:
Molecular Weight:
126.18
Beilstein:
107241
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
208-210 °C/748 mmHg (lit.)
mp
57-62 °C (lit.)
SMILES string
CC(=O)c1ccsc1
InChI
1S/C6H6OS/c1-5(7)6-2-3-8-4-6/h2-4H,1H3
InChI key
RNIDWJDZNNVFDY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Acetylthiophene modified glassy carbon electrode has been used in voltammetric determination of uric acid in urine samples.
Application
3-Acetylthiophene was used in the preparation of 1-(methylthiophenylidine)-8-naphthylamine(Schiff′s base) and 1-(thiophen-3-yl)ethanone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Electro-oxidative polymerization of Schiff-base of 1, 8-diaminonaphthaline and 3-acetylthiophene. I. Preparation and study the redox behaviour of the resulting polymer.
Hathoot AA.
Eur. Polymer J., 36(5), 1063-1071 (2000)
Mehmet Aslanoglu et al.
Chemical & pharmaceutical bulletin, 56(3), 282-286 (2008-03-04)
A reliable and reproducible method for the determination of uric acid in urine samples has been developed. The method is based on the modification of a glassy carbon electrode by 3-acetylthiophene using cyclic voltammetry. The poly(3-acetylthiophene) modified glassy carbon electrode
Ewa Rozycka-Sokolowska et al.
Acta crystallographica. Section C, Crystal structure communications, 67(Pt 6), o209-o211 (2011-06-03)
The structure of the title compound, C(6)H(6)OS, exhibits a flip-type disorder of the thiophene ring [occupancy ratio = 0.848 (3):0.152 (3)], which is typical for many thiophene derivatives. The puckered thiophene ring is essentially coplanar with the plane formed by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service