194530
Hexanoic anhydride
97%
Synonym(s):
Caproic anhydride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
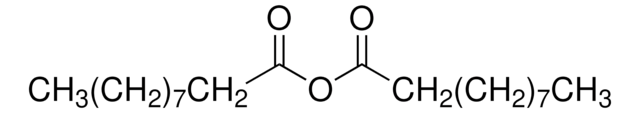
[CH3(CH2)4CO]2O
CAS Number:
Molecular Weight:
214.30
Beilstein:
1776561
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.428 (lit.)
bp
246-248 °C (lit.)
density
0.928 g/mL at 20 °C (lit.)
functional group
anhydride
ester
SMILES string
CCCCCC(=O)OC(=O)CCCCC
InChI
1S/C12H22O3/c1-3-5-7-9-11(13)15-12(14)10-8-6-4-2/h3-10H2,1-2H3
InChI key
PKHMTIRCAFTBDS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Hexanoic anhydride was used in:
- green synthesis of esters of acyclovir (acyclovir prodrugs)
- preparation of hexanoyl-modified chitosan nanoparticles
- preparation of chitosan-based polymeric surfactants via N-acylation of chitosans
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kashappa Goud Desai et al.
Drug delivery, 13(5), 375-381 (2006-08-01)
Hexanoyl chitosan was synthesized through a coupling reaction between chitosan and hexanoic anhydride. Proton nuclear magnetic resonance (1HNMR) and fourier-transform infrared (FTIR) spectroscopy studies showed the formation of hexanoyl chitosan. The nanoparticles of hexanoyl chitosan were prepared through ionotropic gelation
Moo-Yeal Lee et al.
International journal of biological macromolecules, 36(3), 152-158 (2005-07-14)
Chitosan-based polymeric surfactants (CBPSs) were prepared by N-acylation of chitosans (chitosan 10 and 500) with several acid anhydrides such as hexanoic (C6), lauric (C12), and palmitic (C16) anhydrides. Among the CBPS samples, CBPSs having a good solubility at pH 4.0
Rubén de Regil-Hernández et al.
Chemical & pharmaceutical bulletin, 59(9), 1089-1093 (2011-09-02)
Different green synthesis of alkyl esters of acyclovir (acyclovir prodrugs) is described. Hexanoic, decanoic, dodecanoic and tetradecanoic acyclovir esters were synthesized reacting acyclovir and the respective acid anhydride in dimethyl sulfoxide (DMSO), in solvents from renewable sources and without solvent
Yeon-Ji Hong et al.
Journal of biomaterials science. Polymer edition, 26(12), 766-779 (2015-06-11)
Complexation-triggerable liposomes were prepared by modifying the surface of egg phosphatidylcholine (EPC) liposomes with hydrophobicized silk fibroin (HmSF) and hydrophobicized chitosan (HmCh). Maximum complexation, determined by measuring the diameter of complexation, was found when the ratio of HmSF to HmCh
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service