All Photos(1)
About This Item
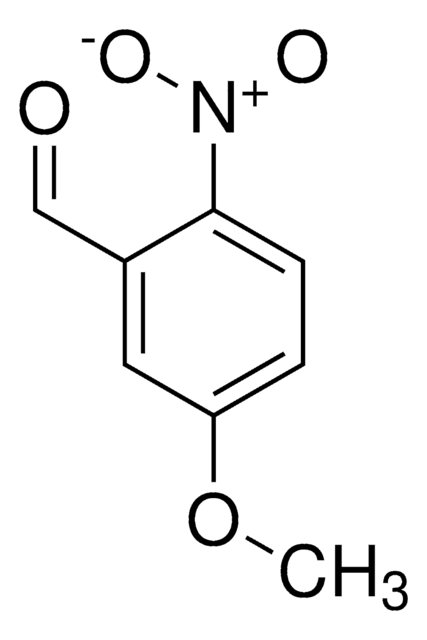
Linear Formula:
CH3OC6H3(NO2)CHO
CAS Number:
Molecular Weight:
181.15
Beilstein:
1959385
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97%
form
solid
mp
97-101 °C (lit.)
functional group
aldehyde
nitro
SMILES string
[H]C(=O)c1cccc(OC)c1[N+]([O-])=O
InChI
1S/C8H7NO4/c1-13-7-4-2-3-6(5-10)8(7)9(11)12/h2-5H,1H3
InChI key
GDTUACILWWLIJF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Methoxy-2-nitrobenzaldehyde undergoes 1,4-diazabicyclo[2.2.2]octane-catalyzed reaction with methyl vinyl ketone (MVK) to afford normal Baylis-Hillman adduct, the MVK dimer and a pair of diastereomeric bis-(MVK)Baylis-Hillman adducts.
Application
3-Methoxy-2-nitrobenzaldehyde was used in the synthesis of 8-hydroxyquinazoline, methy-3-methoxyanthranilate and 3-methoxy-2-nitrobenzylidenebisformamide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Elucidation of the complex Baylis-Hillman reaction of 3-methoxy-2-nitrobenzaldehyde with methyl vinyl ketone.
Idahosa KC, et al.
South African Journal of Chemistry, 64, 144-150 (2011)
972. Analogues of 8-hydroxyquinoline having additional cyclic nitrogen atoms. Part I. Preparative.
Albert A and Hampton A.
Journal of the Chemical Society, 4985-4993 (1952)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service