130648
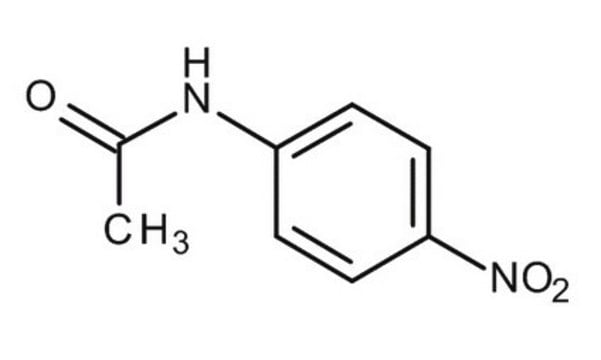
4-Nitroacetanilide
98%
Synonym(s):
4′-Nitroacetanilide, Acetic acid 4-nitroanilide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CONHC6H4NO2
CAS Number:
Molecular Weight:
180.16
Beilstein:
2211962
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
213-215 °C (lit.)
functional group
amide
SMILES string
CC(=O)Nc1ccc(cc1)[N+]([O-])=O
InChI
1S/C8H8N2O3/c1-6(11)9-7-2-4-8(5-3-7)10(12)13/h2-5H,1H3,(H,9,11)
InChI key
NQRLPDFELNCFHW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Nitroacetanilide was used as a test substrate and its hydrolysis was determined by UV spectroscopic measurements. It was also used to prepare 4-aminoacetanilide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shinji Takenaka et al.
Journal of bioscience and bioengineering, 107(1), 27-32 (2009-01-17)
Bacillus cereus strain 10-L-2 synthesizes two arylamine N-acetyltransferases (Nat-a and Nat-b) with broad substrate specificities toward aniline and its derivatives. In southern blot analysis using probes encoding the NH2-terminus of Nat-b and a conserved region of N-acetyltransferases, digested total DNA
María F Montenegro et al.
Biological chemistry, 389(4), 425-432 (2008-01-23)
Apart from its esterase activity, butyrylcholinesterase (BuChE) displays aryl acylamidase (AAA) activity able to hydrolyze o-nitroacetanilide (ONA) and its trifluoro-derivative (F-ONA). We report here that, despite amidase and esterase sites residing in the same protein, in human samples depleted of
Dirk Volkmer et al.
Inorganic chemistry, 35(13), 3792-3803 (1996-06-19)
Dinuclear nickel(II) complexes of the ligands 2,6-bis[bis((2-benzimidazolylmethyl)amino)methyl]-p-cresol (bbapOH), N,N,N',N'-tetrakis(2-benzimidazolylmethyl)-2-hydroxy-1,3-diaminopropane (tbpOH), N-methyl-N,N',N'-tris(2-benzimidazolylmethyl)-2-hydroxy-1,3-diaminopropane (m-tbpOH) and 1-[N,N-bis(2-benzimidazolylmethyl)amino]-3-[2-(3,5-dimethyl-1H-pyrazol-1-yl)ethoxy]-2-hydroxypropane (bpepOH) were prepared in order to model the active site of urease. The novel asymmetric structures of the dinuclear complexes were characterized by X-ray structure analysis.
Short-column liquid chromatographic assay for caffeine and chloramphenicol in serum.
R S Markin et al.
Journal of chromatography, 525(2), 464-470 (1990-02-23)
Ross L Stein
Biochemistry, 41(3), 991-1000 (2002-01-16)
Aryl acylamidase (EC 3.1.5.13; AAA) catalyzes the hydrolysis of p-nitroacetanilide (PNAA) via the standard three-step mechanism of serine hydrolases: binding of substrate (K(s)), acylation of active-site serine (k(acyl)), and hydrolytic deacylation (k(deacyl)). Key mechanistic findings that emerged from this study
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service