112755
DBE-4 dibasic ester
98%
Synonym(s):
Dimethyl succinate, Succinic acid dimethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
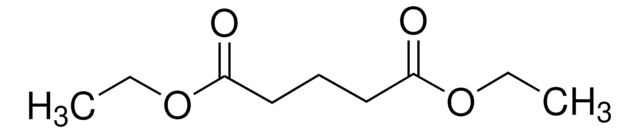
CH3OCOCH2CH2COOCH3
CAS Number:
Molecular Weight:
146.14
Beilstein:
956776
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
0.3 mmHg ( 20 °C)
Quality Level
Assay
98%
autoignition temp.
689 °F
expl. lim.
8.5 %
refractive index
n20/D 1.419 (lit.)
bp
200 °C (lit.)
mp
16-19 °C (lit.)
density
1.117 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
COC(=O)CCC(=O)OC
InChI
1S/C6H10O4/c1-9-5(7)3-4-6(8)10-2/h3-4H2,1-2H3
InChI key
MUXOBHXGJLMRAB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Dimethyl succinate (DBE-4 dibasic ester ) is obtained by the oxidation of 1,4-butanediol and butyrolactone by using supported gold, palladium and gold-palladium nanoparticles.
DBE-4 can be used as an intermediate in the preparation of 1,4-butanediol, γ-butyrolactone, tetrahydrofuran and itaconic acid.
DBE-4 can be used as an intermediate in the preparation of 1,4-butanediol, γ-butyrolactone, tetrahydrofuran and itaconic acid.
Legal Information
DuPont product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
201.2 °F - closed cup
Flash Point(C)
94 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Determination and correlation of ternary isobaric vapour-liquid equilibrium data of (dimethyl succinate+ dimethyl glutarate+ dimethyl adipate) at 2, 5 and 8 kPa
Zhi C, et al.
The Journal of Chemical Thermodynamics, 143, 106047-106047 (2020)
Gemma L Brett et al.
ChemSusChem, 6(10), 1952-1958 (2013-10-10)
The oxidation of 1,4-butanediol and butyrolactone have been investigated by using supported gold, palladium and gold-palladium nanoparticles. The products of such reactions are valuable chemical intermediates and, for example, can present a viable pathway for the sustainable production of polymers.
L Ladriere et al.
JPEN. Journal of parenteral and enteral nutrition, 20(4), 251-256 (1996-07-01)
Succinic acid dimethyl ester (SAD) is efficiently metabolized in several cell types as pancreatic islet cells, hepatocytes, and colonocytes. The purpose of this study was to assess the overall nutritional value of SAD in the whole organism. SAD was infused
L Ladrière et al.
Metabolism: clinical and experimental, 48(1), 102-106 (1999-01-27)
The metabolism of [2,3-13C]succinic acid dimethyl ester ([2,3-13C]-SAD) 10 mmol/L was examined in hepatocytes from overnight-fasted normal rats, 3-day starved rats, and overnight-fasted hereditarily diabetic Goto-Kakizaki (GK) rats. The amount of 13C-labeled succinate, fumarate, malate, lactate, alanine, and aspartate released
V Leclercq-Meyer et al.
Life sciences, 58(14), 1195-1199 (1996-01-01)
The glucagon-like peptide 1 (7-36) amide (GLP-1, 1.0 nM) was administered to isolated rat pancreases perfused either in the absence of exogenous nutrient or presence of 10 mM succinic acid dimethyl ester (SAD). In the absence of any exogenous nutrient
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service