All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C35H59N11O5
Molecular Weight:
713.91
UNSPSC Code:
12352200
NACRES:
NA.32
Recommended Products
Assay
≥95% (HPLC)
form
lyophilized
composition
Peptide Content, ≥55%
storage condition
protect from light
storage temp.
−20°C
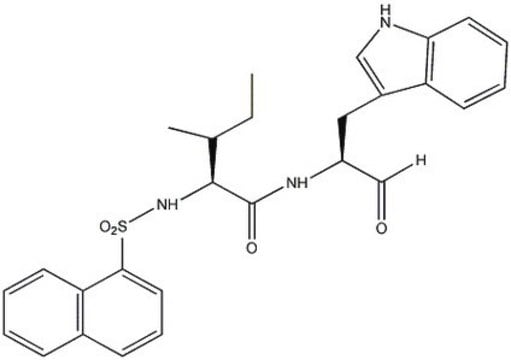
Amino Acid Sequence
Arg-Lys-Leu-Leu-Trp-NH2
General description
Cathepsin L Inhibitor is a histone H3-processing enzyme. It is important for maintaining epidermal homeostasis, regular hair follicle morphogenesis and cycling. Cathepsin L is involved in protein degradation. It might regulate normal functioning of the immune system. Cathepsin L regulates the death of macrophages, necrotic core formation and development of atherosclerotic plaque instability.
Application
Cathepsin L is a lysosomal cysteine proteinase that metabolizes collagens and elastins. The roles and activity of Cathepsin L can be studied with the aid of peptide inhibitors such as the pentapeptide amide RKLLW-NH2 (Arg-Lys-Leu-Leu-Trp-NH2).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Brinker et al.
European journal of biochemistry, 267(16), 5085-5092 (2000-08-10)
By screening a combinatorial pentapeptide amide collection in an inhibition assay, we systematically evaluated the potential of 19 proteinogenic amino acids and seven nonproteinogenic amino acids to serve as building blocks for inhibitors of human cathepsin L. Particularly efficient were
Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells
Liu J, et al.
Atherosclerosis, 184(2), 302-311 (2006)
Protease signalling: the cutting edge
Turk B, et al.
The Embo Journal, 31(7), 1630-1643 (2012)
Cathepsin L is significantly associated with apoptosis and plaque destabilization in human atherosclerosis
Li W, et al.
Atherosclerosis, 202(1), 92-102 (2009)
V Turk et al.
The EMBO journal, 20(17), 4629-4633 (2001-09-05)
From their discovery in the first half of the 20th century, lysosomal cysteine proteases have come a long way: from being the enzymes non-selectively degrading proteins in lysosomes to being those responsible for a number of important cellular processes. Some
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service