M70800
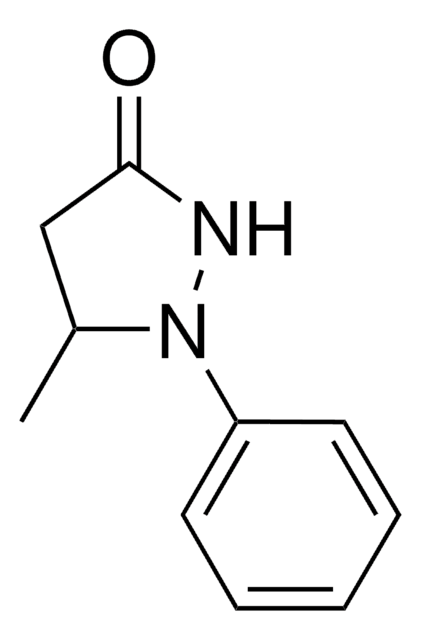
3-Methyl-1-phenyl-2-pyrazoline-5-one
99%, for peptide synthesis
Synonym(s):
Edaravone, 5-Methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
About This Item
Recommended Products
product name
3-Methyl-1-phenyl-2-pyrazoline-5-one, 99%
Assay
99%
form
powder, crystals or chunks
bp
287 °C/265 mmHg (lit.)
mp
126-128 °C (lit.)
application(s)
peptide synthesis
SMILES string
CC1=NN(C(=O)C1)c2ccccc2
InChI
1S/C10H10N2O/c1-8-7-10(13)12(11-8)9-5-3-2-4-6-9/h2-6H,7H2,1H3
InChI key
QELUYTUMUWHWMC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- As a reagent for the detection of reducing carbohydrates by ESI/MALDI-MS.
- To improve the sensitivity of reducing mono- and oligo-saccharides for their subsequent determination using capillary zone electrophoresis.
- For the precolumn derivatization of the monosaccharide components of Salvia miltiorrhiza, liguspyragine hydrochloride, and glucose injection prior to their analysis by high performance liquid chromatography (HPLC).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Glycans play a key role in protein structure and disease; representation on cell surfaces is the glycome.
Glycans play a key role in protein structure and disease; representation on cell surfaces is the glycome.
Glycans play a key role in protein structure and disease; representation on cell surfaces is the glycome.
Glycans play a key role in protein structure and disease; representation on cell surfaces is the glycome.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service