851450
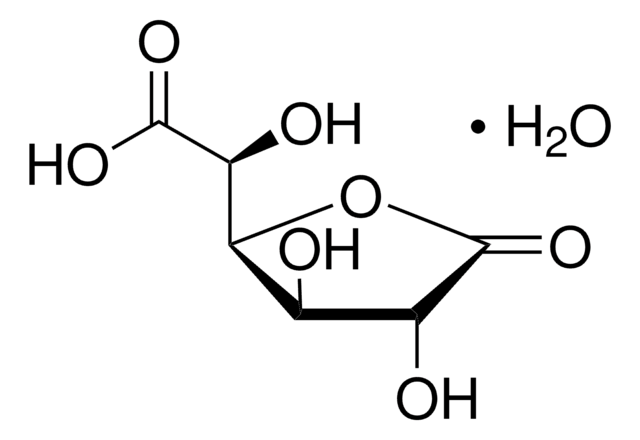
D-(+)-Glucuronic acid γ-lactone
≥99%
Synonym(s):
D-(+)-Glucurono-6,3-lactone, D-Glucurone, D-Glucurono-6,3-lactone, Glucuronolactone
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H8O6
CAS Number:
Molecular Weight:
176.12
Beilstein:
83595
EC Number:
MDL number:
UNSPSC Code:
12352115
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
powder
optical activity
[α]24/D +18.8°, c = 8 in H2O
mp
172-175 °C (lit.)
solubility
water: soluble 25 mg/mL, clear, colorless
SMILES string
O=C([C@@H]([C@@H](O1)[C@H](O)[C@H](O)C1=O)O)[H]
InChI
1S/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h1-5,8-10H/t2-,3+,4-,5+/m0/s1
InChI key
UYUXSRADSPPKRZ-SKNVOMKLSA-N
Looking for similar products? Visit Product Comparison Guide
General description
D-(+)-Glucuronic acid γ-lactone (Glucourono-γ-lactone, Glucurone or Glycurone) is a carbohydrate derivative. It converted into L-ascorbic acid in animals and human body. Its molecule contains two five-membered rings. Its crystal structure has been studied.
Application
D-(+)-Glucuronic acid γ-lactone may be used in the following studies:
- As starting ragent in the synthesis of 2,3,4,-tris(tert.-butyldimethysilyl) glucuronic acid trichloroethylester, required for the preparation of 1-O-acyl glucuronide of the anti-inflammatory drug ML-3000.
- Synthesis of optically active glucopyranoses.
- Synthesis of long-chain alkyl glucofuranosides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L A Reyner et al.
Physiology & behavior, 75(3), 331-335 (2002-03-19)
Driver sleepiness is a major cause of serious road crashes. Coffee is often used as an effective countermeasure to driver sleepiness. However, the caffeine levels in coffee are variable, whereas certain proprietary "functional energy drinks" (FEDs) contain known levels of
P Florio et al.
Carbohydrate research, 328(4), 445-448 (2000-11-28)
A concise route to novel mimetics of Kdn2en, based on delta4-uronic acids, from D-glucurono-6,3-lactone is presented. Uronic acid-based mimetics in which an aliphatic ether (O-glycoside), a thioether (S-glycoside), or acetamide takes the place of the natural C-6 glycerol sidechain of
Rongchun Wang et al.
Bioscience, biotechnology, and biochemistry, 74(3), 601-605 (2010-03-09)
The degradation kinetics of glucuronic acid (GlcA) under subcritical conditions from 160 to 200 degrees C was studied in a continuous tubular reactor. The formation of glucuronolactone (GlcL) during the treatment of GlcA in subcritical water was substantiated by ESI-TOF-MS
Chemical & Pharmaceutical Bulletin, 41, 1197-1197 (1993)
The crystal structure of beta-D-glucurono-gamma-lactone.
S H Kim et al.
Acta crystallographica, 22(5), 733-743 (1967-05-10)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service