All Photos(1)
About This Item
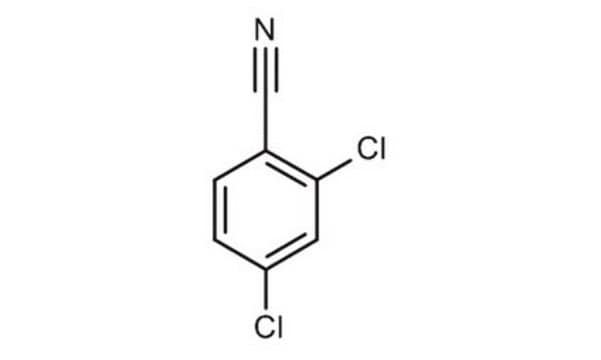
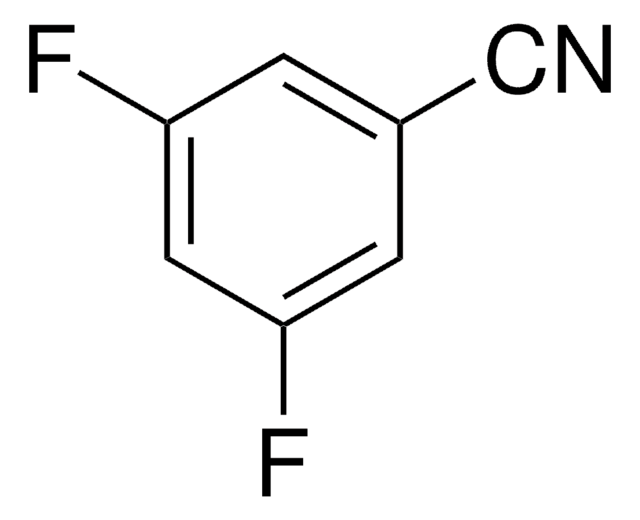
Linear Formula:
Cl2C6H3CN
CAS Number:
Molecular Weight:
172.01
Beilstein:
2326352
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
74-78 °C (lit.)
functional group
chloro
nitrile
SMILES string
Clc1ccc(cc1Cl)C#N
InChI
1S/C7H3Cl2N/c8-6-2-1-5(4-10)3-7(6)9/h1-3H
InChI key
KUWBYWUSERRVQP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3,4-Dichlorobenzonitrile (3,4-DCBN) is an aromatic nitrile that can be prepared from 3,4-dichlorobenzamide.
Application
3,4-Dichlorobenzonitrilemay be used in the preparation of:
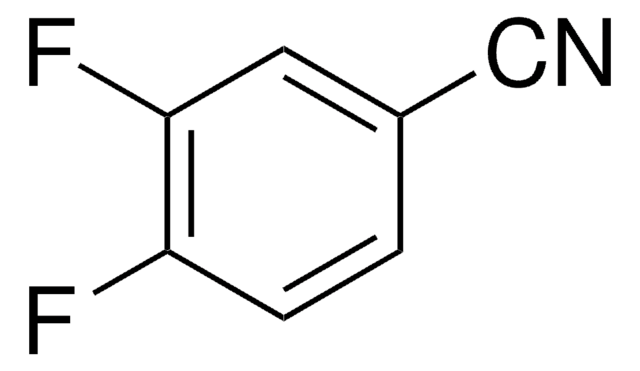
- 3,4-difluorobenzonitrile
- 3-chloro-4-fluorobenzonitrile
- 5-chloromethyl-3-( 3,4-dichlorophenyl)isoxazole
- N-[3-(2-pyridyl)isoquinolin-1-yl]-3,4-dichlorobenzamidine
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
3-Aryl-5-halomethylisoxazoles. A new class of anthelmintics.
Sen HG, et al.
Journal of Medicinal Chemistry, 9(3), 431-433 (1966)
Synthesis and copper-dependent antimycoplasmal activity of 1-amino-3-(2-pyridyl) isoquinoline derivatives. 2. Amidines.
De Zwart MAH, et al.
Journal of Medicinal Chemistry, 32(2), 487-493 (1989)
Wouter Huiting et al.
eLife, 11 (2022-02-25)
A loss of the checkpoint kinase ataxia telangiectasia mutated (ATM) leads to impairments in the DNA damage response, and in humans causes cerebellar neurodegeneration, and an increased risk of cancer. A loss of ATM is also associated with increased protein
Synthesis of 3, 4-difluorobenzonitrile and monofluorobenzonitriles by means of halogen-exchange fluorination.
Suzuki H and Kimura Y.
Journal of Fluorine Chemistry, 52(3), 341-351 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service