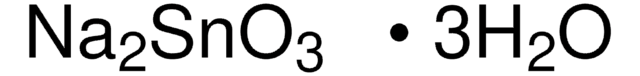379387
Dithiooxamide
97%
Synonym(s):
Dithiooxalic diamide, Rubeanic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2CSCSNH2
CAS Number:
Molecular Weight:
120.20
Beilstein:
605577
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
≥300 °C (lit.)
solubility
ethanol: soluble 40 mg/10 mL, clear, red
functional group
amine
SMILES string
NC(=S)C(N)=S
InChI
1S/C2H4N2S2/c3-1(5)2(4)6/h(H2,3,5)(H2,4,6)
InChI key
OAEGRYMCJYIXQT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Dithiooxamide is reported to form complexes with Ni(II).
Application
Dithiooxamide may be used in the following studies:
- Synthesis of thiazolothiazole-linked porous organic polymers under solvothermal conditions.
- As modifier to prepare the modified glassy carbon electrode, used to investigate the electrochemical properties of quercetin, an important flavonoid derivative.
- Synthesis of new chelating resin of dithiooxamide (rubeanic acid)-formaldehyde (DTOF), used in separation and concentration of silver ions.
- Synthesis of N,N′-disubstituted dithiooxamides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Nickel (II) complexes with dithiooxamide, N, N'-di-methyl-and N, N'-di-hydroxyethyl-dithiooxamide.
Peyronel G, et al.
Inorgorganica Chimica Acta, 5, 627-633 (1971)
Preparation of Dithiooxamide Derivatives.
Hurd RN, et al.
The Journal of Organic Chemistry, 26(10), 3980-3987 (1961)
Xiang Zhu et al.
Chemical communications (Cambridge, England), 50(95), 15055-15058 (2014-10-21)
Thiazolothiazole-linked porous organic polymers have been synthesized from a facile catalyst-free condensation reaction between aldehydes and dithiooxamide under solvothermal conditions. The resultant porous frameworks exhibit a highly selective uptake of CO2 over N2 under ambient conditions.
S Goto et al.
The Japanese journal of surgery, 14(2), 135-138 (1984-03-01)
An improved staining technique for acetylcholinesterase (AChE) activity using rubeanic acid was used to make a clinical diagnosis in 54 children with constipation. Nineteen were thus confirmed to have Hirschsprung's disease. False positive or negative reactions were nil. The sites
H O Desseyn et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 59(6), 1359-1372 (2003-03-28)
The article describes the vibrational characterization of the secondary thioamide formation and especially a spectroscopical interesting study of different types of hydrogen bonding in NN'-dihydroxyalkyldithiooxamides.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service