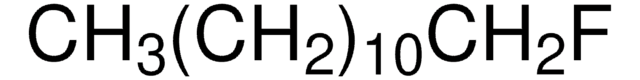All Photos(1)
About This Item
Linear Formula:
CH3(CH2)7F
CAS Number:
Molecular Weight:
132.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.396 (lit.)
bp
142-146 °C (lit.)
density
0.814 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCF
InChI
1S/C8H17F/c1-2-3-4-5-6-7-8-9/h2-8H2,1H3
InChI key
DHIVLKMGKIZOHF-UHFFFAOYSA-N
General description
1-Fluorooctane undergoes C-F bond-cleavage reaction with phenyl magnesium chloride to give n-octylbenzene. It reacts rapidly with trimethylsilyl iodide to give corresponding octyl iodides and trimethylsilyl fluoride.
Application
1-Fluorooctane has been used as a molecular probe in evaluations of gas chromatographic stationary phases consisting of bromo- and chloro-derivatives of a C78 branched alkane.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
107.6 °F - closed cup
Flash Point(C)
42 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ervin Sz Kováts et al.
Journal of chromatography. A, 1113(1-2), 206-219 (2006-02-25)
In a paper published in 1992 [K.S. Reddy, J.-Cl. Dutoit, E.sz. Kováts, Pair-wise interactions by gas chromatography. I. Interaction free enthalpies of solutes with non-associated primary alcohol groups, J. Chromatogr. 609 (1992) 229] retention indices and standard chemical potential differences
Kouki Matsubara et al.
Organic letters, 11(8), 1765-1768 (2009-03-14)
An unexpected C-F bond-cleavage reaction of unactivated fluoroalkanes with the well-known Grignard reagents without using metal catalysts has been discovered. For example, a reaction between 1-fluorooctane and phenyl magnesium chloride gave n-octylbenzene in moderate yield. This coupling reaction via the
Halogen redistribution reactions between alkyl halides and trimethylsilyl iodide.
Friedrich EC and De Lucca G.
Journal of Organometallic Chemistry, 226(2), 143-148 (1982)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service