추천 제품
Grade
pharmaceutical primary standard
API family
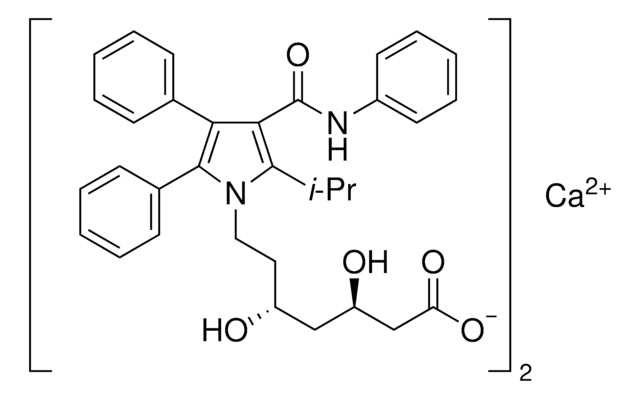
atorvastatin
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
InChI
1S/2C33H35FN2O5.Ca.3H2O/c2*1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40;;;;/h2*3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40);;3*1H2/q;;+2;;;/p-2/t2*26-,27-;;;;/m11..../s1
InChI key
SHZPNDRIDUBNMH-NIJVSVLQSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Atorvastatin Calcium is a synthetic selective inhibitor of HMG-CoA reductase. It is insoluble in aqueous solution with pH below 4 and is freely soluble in methanol.
Atorvastatin Calcium is a synthetic selective inhibitor of HMG-CoA reductase. It is insoluble in aqueous solution with pH below 4 and is freely soluble in methanol.
애플리케이션
Atorvastatin calcium USP reference standard intended for use in specified quality tests and assays.
Also used to prepare system suitability, standard, system suitability stock, identification, and standard stock solution for assay and impurity analysis according to the given below monographs of United States Pharmacopeia (USP):
Also used to prepare system suitability, standard, system suitability stock, identification, and standard stock solution for assay and impurity analysis according to the given below monographs of United States Pharmacopeia (USP):
- Atorvastatin Calcium
- Atorvastatin Calcium Tablets
- Amlodipine and Atorvastatin Tablets
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Carc. 2 - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Atorvastatin Calcium Tablets
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 42(1), 418-418 (2020)
Atorvastatin and Atorvastatin Tablets
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 43(2), 265-265 (2020)
Atorvastatin Calcium
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 35(1), 414-414 (2022)
Yanli Wang et al.
Histology and histopathology, 29(12), 1593-1600 (2014-08-01)
Statins are often prescribed for treatment of cardiovascular diseases, although there are still many patients who cannot be effectively treated by statins alone. Both probucol and cilostazol exhibit anti-atherogenic effects. In the current study, we attempted to investigate whether a
Min-Soo Kim et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 69(2), 454-465 (2008-03-25)
In this work, amorphous atorvastatin calcium nanoparticles were successfully prepared using the supercritical antisolvent (SAS) process. The effect of process variables on particle size and distribution of atorvastatin calcium during particle formation was investigated. Solid state characterization, solubility, intrinsic dissolution
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.