추천 제품
분석
≥98% (HPLC)
형태
solid
색상
off-white
solubility
DMSO: ≥10 mg/mL
H2O: ≥2 mg/mL
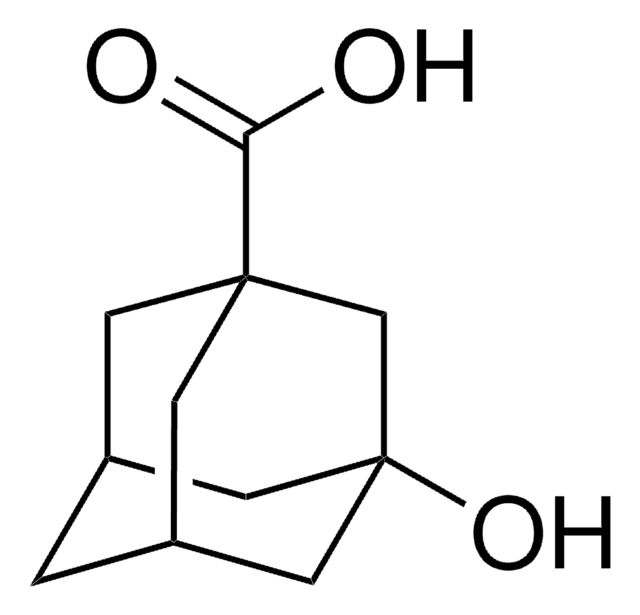
SMILES string
Cl[H].CCCN(CCc1ccccc1)C2CCc3c(O)cccc3C2
InChI
1S/C21H27NO.ClH/c1-2-14-22(15-13-17-7-4-3-5-8-17)19-11-12-20-18(16-19)9-6-10-21(20)23;/h3-10,19,23H,2,11-16H2,1H3;1H
InChI key
XWLCIDLCEZAOEY-UHFFFAOYSA-N
유전자 정보
human ... DRD1(1812) , DRD2(1813) , DRD3(1814) , DRD4(1815) , DRD5(1816)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
생화학적/생리학적 작용
A series of new dopamine (DA) receptor agonists, of the 2-aminotetralin group, i.e. N-0434, N-0437 and N-0734 were investigated in both in vivo and in vitro pharmacological test systems. In vivo, the reversal of the gamma-butyrolactone-induced increase in rat central DOPA biosynthesis rate was taken as a measure of presynaptic activity. The homovanillic acid (HVA) decrease, after intraperitoneal and after oral administration of the drugs was also taken as a measure of presynaptic activity. Postsynaptic activity was measured in two behavioural models, i.e. reserpine reversal and stereotypy induction. The effects of (±)-PPHT (N-0434) these drugs on noradrenaline and dopamine turnover (alpha-MpT method) were studied in addition. The results indicate that all three compounds N-0434 ((±)-PPHT), N-0437 and N-0734 are potent and selective DA agonists that lack significant alpha 2 activity.
Potent D2 dopamine receptor agonist.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
M P Seiler et al.
Journal of medicinal chemistry, 29(6), 912-917 (1986-06-01)
5-Hydroxy-2-aminotetralin derivatives in which one N-alkyl substituent carries a functional group have been prepared and their dopaminergic activities compared with those of 5-hydroxy-2-(di-n-propylamino)tetralin (5-OH-DPAT) and known ergolines. Several members of the series demonstrated high affinities in dopamine (DA) receptor binding
M A Ariano et al.
Brain research, 547(2), 208-222 (1991-05-03)
Selective dopamine receptor ligands, (R,S)-5-(4'-aminophenyl)-8-chloro-2,3,4, 5-tetrahydro-3-methyl-[1H]-3-benzazepin-7-ol, the 4'-amino derivative of the high affinity D1 receptor antagonist SCH 23390, the high affinity D2 receptor antagonist N-(p-aminophenethyl)-spiperone or NAPS, and the D2 selective agonist, 2-(N-phenethyl-N-propyl)-amino-5-hydroxytetralin or PPHT were chemically coupled to the
F J Bosker et al.
European journal of pharmacology, 163(2-3), 319-326 (1989-04-25)
Partial purification of the dopamine D-2 receptor from bovine striatum, solubilized in the presence of 1% digitonin, was obtained by chromatography on wheat germ lectin agarose. The preparation was purified approximately 10-fold. The stability of the receptor preparation was considerably
A Mao et al.
Life sciences, 59(21), PL317-PL324 (1996-01-01)
The concentrations of endogenous ligands generally remain in a bounded range around a basal level, a manifestation of control. The dopaminergic system is an excellent example of a control system in which a negative feedback signal is associated with receptor
T P Burris et al.
Neuroendocrinology, 54(2), 175-183 (1991-08-01)
The ability of low concentrations of dopamine (DA) to stimulate the secretion of prolactin (PRL) was examined in perifused or monolayer cultures of anterior pituitary cells. In cultures perifused with media containing 100 nM DA, changing the DA concentration to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.