C1259
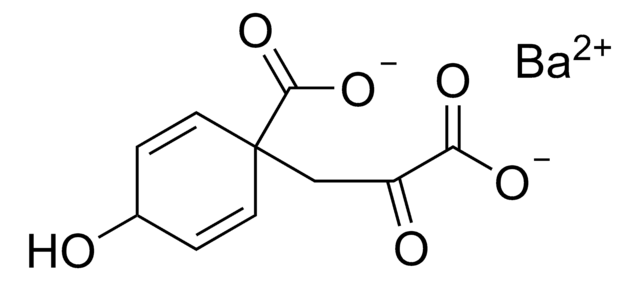
Chorismic acid barium salt from Enterobacter aerogenes
≥55%
동의어(들):
trans-3-([1-Carboxyethenyl]oxy)-4-hydroxy-1,5-cyclohexadiene-1-carboxylic acid
About This Item
추천 제품
형태
powder
Quality Level
농도
≥55%
배송 상태
dry ice
저장 온도
−70°C
SMILES string
[Ba].O[C@@H]1C=CC(=C[C@H]1OC(=C)C(O)=O)C(O)=O
InChI
1S/C10H10O6.Ba.2H/c1-5(9(12)13)16-8-4-6(10(14)15)2-3-7(8)11;;;/h2-4,7-8,11H,1H2,(H,12,13)(H,14,15);;;/t7-,8-;;;/m1.../s1
InChI key
JFTBXNPYKNMOHD-MVYICHOISA-N
애플리케이션
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 2 - STOT SE 3
표적 기관
Eyes,Central nervous system, Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.