모든 사진(1)
About This Item
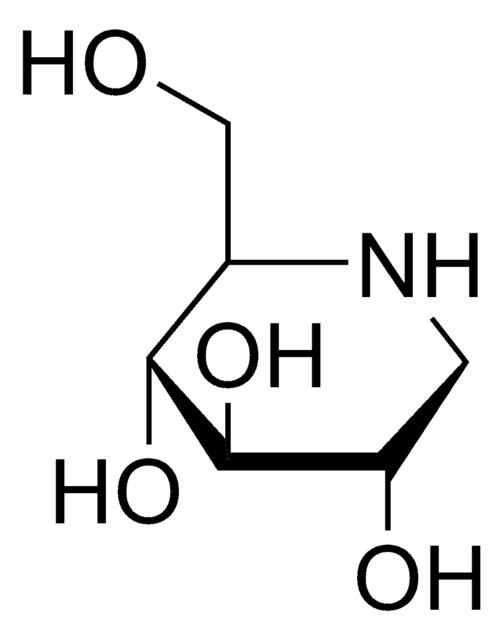
실험식(Hill 표기법):
C7H15NO5
CAS Number:
Molecular Weight:
193.20
UNSPSC 코드:
12352201
PubChem Substance ID:
NACRES:
NA.25
추천 제품
분석
≥98.0% (TLC)
SMILES string
OC[C@H]1N[C@H](CO)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C7H15NO5/c9-1-3-5(11)7(13)6(12)4(2-10)8-3/h3-13H,1-2H2/t3-,4-,5-,6+,7+/m1/s1
InChI key
CLVUFWXGNIFGNC-OVHBTUCOSA-N
애플리케이션
α-Homonojirimycin (HMJ) is used as an inhibitor of several carbohydrate degrading enzymes including α-glucosidases, glycoprotein processing enzyme glucosidase II and maltase.
생화학적/생리학적 작용
α-Homonojirimycin is a potent inhibitor of a range of α-glucosidases, as well as an inhibitor of the glycoprotein processing enzyme glucosidase II.
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
기타 정보
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
K Ikeda et al.
Carbohydrate research, 323(1-4), 73-80 (2000-04-27)
2,6-Dideoxy-7-O-(beta-D-glucopyranosyl) 2,6-imino-D-glycero-L-gulo- heptitol (7-O-beta-D-glucopyranosyl-alpha-homonojirimycin, 1) was isolated from the 50% methanol extract of the whole plant of Lobelia sessilifolia (Campanulaceae), which was found to potently inhibit rice alpha-glucosidase. Adenophorae radix, roots of Adenophora spp. (Campanulaceae), yielded new homonojirimycin derivatives, adenophorine
Gabriel M J Lenagh-Snow et al.
Organic letters, 14(8), 2050-2053 (2012-04-05)
Although there are 32 6-azidoheptitols, there are only 16 homonojirimycin (HNJ) stereoisomers. Two epimeric azidoalditols derived from d-mannose allow the synthesis in water of eight stereoisomers of HNJ.
O R Martin et al.
Bioorganic & medicinal chemistry letters, 9(21), 3171-3174 (1999-11-24)
The structure of a homonojirimycin isomer isolated from Aglaonema treublii and originally proposed as alpha-3,4-di-epi-homonojirimycin was revised to alpha-4-epi-homonojirimycin 3 ("alpha-homoallonojirimycin") on the basis of NMR analysis and synthetic studies. Its activity as a glycosidase inhibitor is compared to that
Shankar D Markad et al.
Bioorganic & medicinal chemistry, 14(16), 5535-5539 (2006-05-10)
Conjugate addition of n-butyl amine to d-glucose derived alpha,beta-unsaturated ester 4 afforded beta-amino esters 5a,b that on reduction of ester group, 1,2-acetonide deprotection, and reductive amination led to the formation of corresponding N-butyl 1-deoxy-D-gluco-homonojirimycin 2c and N-butyl 1-deoxy-L-ido-homonojirimycin 2d which
Chinami Kuriyama et al.
Bioorganic & medicinal chemistry, 16(15), 7330-7336 (2008-07-04)
We investigated in vitro inhibition of mammalian carbohydrate-degrading enzymes by six-membered sugar mimics and their evaluation in cell cultures. 1-Deoxynojirimycin (DNJ) showed no significant inhibition toward glycogen phosphorylase (GP) but was a potent inhibitor of another glycogen-degrading enzyme, amylo-1,6-glucosidase (1,6-GL)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.