Y0001370
Olanzapine
European Pharmacopoeia (EP) Reference Standard
동의어(들):
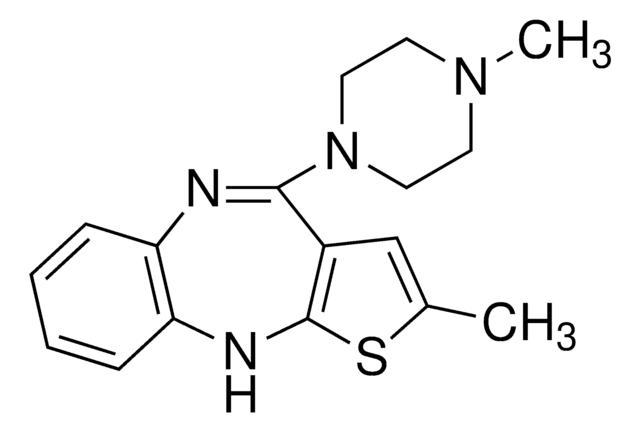
2-Methyl-4-(4-methyl-1-piperazinyl)- 10H-thieno[2,3-b][1,5]benzodiazepine
About This Item
추천 제품
Grade
pharmaceutical primary standard
API family
olanzapine
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
CN1CCN(CC1)C2=Nc3ccccc3Nc4sc(C)cc24
InChI
1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3
InChI key
KVWDHTXUZHCGIO-UHFFFAOYSA-N
유전자 정보
human ... DRD2(1813) , DRD3(1814) , DRD4(1815) , HTR2A(3356) , HTR2C(3358)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
포장
기타 정보
관련 제품
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
표적 기관
Central nervous system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.