추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to Ph. Eur. C0682800
traceable to USP 1097603
API family
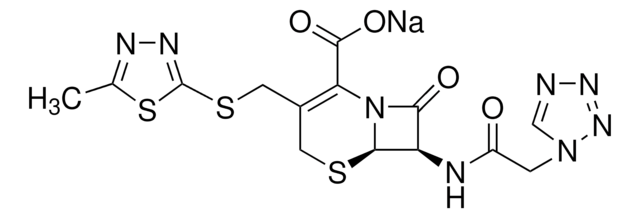
cefazolin
CofA
current certificate can be downloaded
포장
pkg of 500 mg
응용 분야
pharmaceutical
형식
neat
저장 온도
2-8°C
InChI
1S/C14H14N8O4S3/c1-6-17-18-14(29-6)28-4-7-3-27-12-9(11(24)22(12)10(7)13(25)26)16-8(23)2-21-5-15-19-20-21/h5,9,12H,2-4H2,1H3,(H,16,23)(H,25,26)/t9-,12-/m1/s1
InChI key
MLYYVTUWGNIJIB-BXKDBHETSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Cefazolin is a first-generation cephalosporin β-lactam antibiotic drug that exhibits broad-spectrum antimicrobial activity against Klebsiella pneumoniae. It also acts in vitro against Gram-positive aerobic cocci while shows only a limited action on Gram-negative bacteria.
Pharmaceutical secondary standard for application in quality control. Provides pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standard for application in quality control. Provides pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
애플리케이션
Cefazolin has been used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by thin layer chromatography-densitometry technique.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
분석 메모
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
기타 정보
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
각주
To see an example of a Certificate of Analysis for this material enter LRAB0131 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
추천 제품
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
Choose from one of the most recent versions:
이미 열람한 고객
Streamlining methodology for the multiresidue analysis of β -lactam antibiotics in bovine kidney using liquid chromatography-tandem mass spectrometry
Mastovska K and Lightfield AR
Journal of Chromatography A, 1202(2), 118-123 (2008)
Simultaneous identification and quantitative analysis of eight cephalosporins in pharmaceutical formulations by TLC-densitometry
Monika D, et al.
JPC-Journal of Planar Chromatography-Modern TLC, 24(1), 23-29 (2011)
Voltammetric behavior and assay of the antibiotic drug cefazolin sodium in bulk form and pharmaceutical formulation at a mercury electrode
El-Desoky HS, et al.
Journal of Pharmaceutical and Biomedical Analysis, 39(5), 1051-1056 (2005)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.