추천 제품
Grade
pharmaceutical primary standard
API family
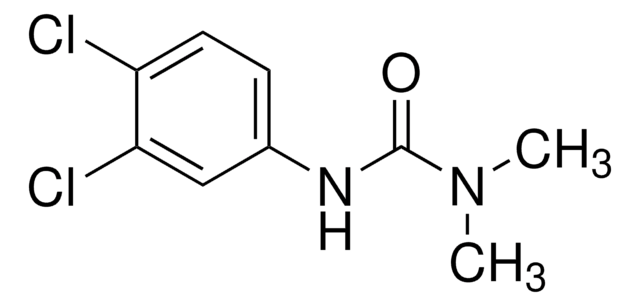
malathion
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
−20°C
InChI
1S/C10H19O6PS2/c1-5-15-9(11)7-8(10(12)16-6-2)19-17(13,14-3)18-4/h8H,5-7H2,1-4H3
InChI key
LPQDGVLVYVULMX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Malathion impurity A EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
141.8 °F
Flash Point (°C)
61 °C
가장 최신 버전 중 하나를 선택하세요:
T R Fukuto
Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes, 18(1), 89-117 (1983-01-01)
Impurities such as O,S,S-trimethyl phosphorodithioate (TMPD) and the S-methyl isomer of malathion (isomalathion) strongly potentiated the mammalian toxicity of malathion. In contrast, impurities present in the phosphoramidothioate insecticide acephate had an antagonizing effect on its mammalian toxicity. The potentiation of
B Clothier et al.
Biochimica et biophysica acta, 660(2), 306-316 (1981-08-13)
The reaction of bovine erythrocyte acetylcholinesterase (acetylcholine acetylhydrolase, EC 3.1.1.7) with a set of structurally related phosphorothiolates was studied in order to investigate the properties of the phosphorylated enzymes and to identify the leaving group. OOS- and OOS-trimethyl phosphorothiolates and
C E Berkman et al.
Chemical research in toxicology, 6(5), 724-730 (1993-09-01)
The biomolecular reaction constants (ki), dissociation constants (Kd), and phosphorylation constants (kp) were determined for the enantiomers of malaoxon against rat brain acetylcholinesterase, and for the stereoisomers of isomalathion against rat brain acetylcholinesterase and electric eel acetylcholinesterase. (R)-Malaoxon was an
Franca M Buratti et al.
Journal of biochemical and molecular toxicology, 19(6), 406-414 (2006-01-20)
The organophosphorothioate (OPT) pesticide malathion (MAL) in mammals is readily hydrolyzed by mammalian carboxylesterases (CE). The reaction competes with the CYP-catalyzed formation of malaoxon (MOX), the toxic metabolite. Alterations or individual variations in CE activity may result in increased MOX
Jonathan A Doorn et al.
Chemical research in toxicology, 16(8), 958-965 (2003-08-20)
The present study was undertaken to test the hypothesis that acetylcholinesterase (AChE) inhibition by isomalathion stereoisomers proceeds with different primary leaving groups for (1R)- and (1S)-isomers. Consistent with results obtained with enzyme from other species, AChE from Torpedo californica (TcAChE)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.