51964
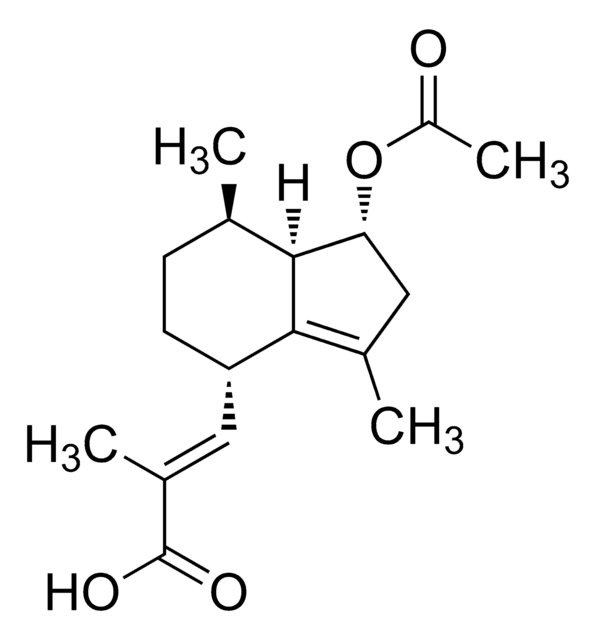
Valerenic acid
analytical standard
동의어(들):
(2E)-3-[(4S,7R,7aR)-2,4,5,6,7,7a-Hexadydro-3,7-dimethyl- 1H-inden-4-yl]-2-methyl-2-propenoic acid
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C15H22O2
CAS Number:
Molecular Weight:
234.33
Beilstein:
3138020
MDL number:
UNSPSC 코드:
85151701
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
analytical standard
Quality Level
분석
≥98.0% (HPLC)
유통기한
limited shelf life, expiry date on the label
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
cleaning products
cosmetics
food and beverages
personal care
형식
neat
저장 온도
−20°C
SMILES string
[H][C@]12CCC(C)=C1[C@@H](CC[C@H]2C)\C=C(/C)C(O)=O
InChI
1S/C15H22O2/c1-9-4-6-12(8-11(3)15(16)17)14-10(2)5-7-13(9)14/h8-9,12-13H,4-7H2,1-3H3,(H,16,17)/b11-8+/t9-,12+,13-/m1/s1
InChI key
FEBNTWHYQKGEIQ-SUKRRCERSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Valerenic acid is a bioactive sesquiterpene and a natural product, isolated from Valeriana officinalis type of plant. It can find applications in the treatment of several dis-functions of the central nervous system, since it is potent modulator of the GABAA receptor.
애플리케이션
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
기타 정보
Constituent of Valeriana officinalis
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
이미 열람한 고객
P J Houghton
The Journal of pharmacy and pharmacology, 51(5), 505-512 (1999-07-20)
The underground organs of members of the genus Valeriana (Valerianaceae), as well as related genera such as Nardostachys, are used in the traditional medicine of many cultures as mild sedatives and tranquillizers and to aid the induction of sleep. V.
Highly potent modulation of GABA(A) receptors by valerenic acid derivatives.
Sascha Kopp et al.
ChemMedChem, 5(5), 678-681 (2010-03-18)
Takashi Kitayama et al.
Bioscience, biotechnology, and biochemistry, 74(9), 1963-1964 (2010-09-14)
A concise synthesis of valerena-4,7(11)-diene with potent sedative activity was achieved in three steps involving, reduction of carboxylic acid, bromination of the resulting alcohol, and reduction of the bromide from valerenic acid in a 63% total yield. This synthetic method
Juergen Ramharter et al.
Organic letters, 13(19), 5310-5313 (2011-09-08)
A mild and selective one-pot procedure to provide 2,4-dienols from simple cycloalkenones in high yields is described. This transformation is based on the in situ formation of acid-labile allylic alcohols, which on treatment with trifluoroacetic acid undergo a formal [1,3]-hydroxy
Dietmar Benke et al.
Neuropharmacology, 56(1), 174-181 (2008-07-08)
Valerian extracts have been used for centuries to alleviate restlessness and anxiety albeit with unknown mechanism of action in vivo. We now describe a specific binding site on GABA(A) receptors with nM affinity for valerenic acid and valerenol, common constituents
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.