935417
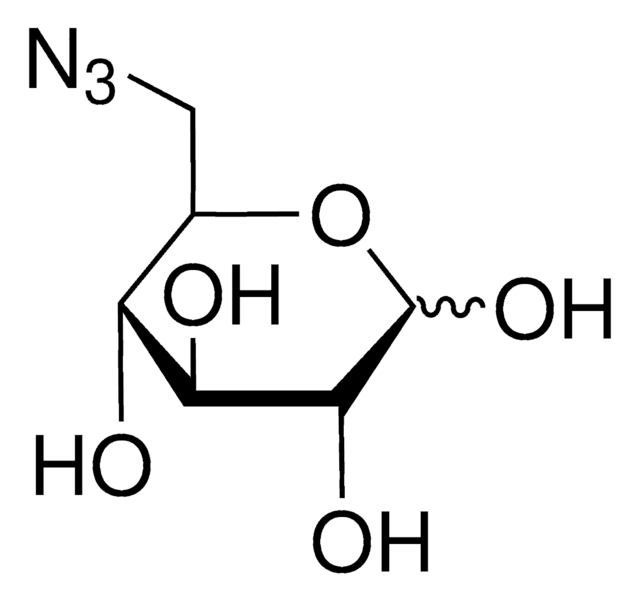
β-D-Glucopyranosyl azide
≥95%
동의어(들):
2-Acetamido-2-deoxy-3,4,6-tri-O-acetyl-β-D-glucopyranosyl azide, 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-D-glucopyranosyl azide, Glucopyranosyl azide, 2-acetamido-2-deoxy-, 3,4,6-triacetate, β-D-, Glucopyranosyl azide, 2-acetamido-2-deoxy-, triacetate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C14H20N4O8
CAS Number:
Molecular Weight:
372.33
MDL number:
UNSPSC 코드:
12352100
NACRES:
NA.21
추천 제품
Quality Level
분석
≥95%
양식
powder or crystals
색상
white to light yellow
bp
501.81 °C
mp
128-130 °C
density
1.30 g/mL
저장 온도
−20°C
SMILES string
[N-]=[N+]=NC1OC(COC(=O)C)C(OC(=O)C)C(OC(=O)C)C1NC(=O)C
InChI
InChI=1S/C14H20N4O8/c1-6(19)16-11-13(25-9(4)22)12(24-8(3)21)10(5-23-7(2)20)26-14(11)17-18-15/h10-14H,5H2,1-4H3,(H,16,19)/t10-,11-,12-,13-,14-/m1/s1
InChI key
RMCFMPMNMQZHSF-DHGKCCLASA-N
애플리케이션
β-D-Glucopyranosyl azide, 2-(acetylamino)-2-deoxy-, 3,4,6-triacetate is commonly used in organic synthesis, particularly for the preparation of glycosyl azides, which are versatile intermediates in the synthesis of glycoconjugates. In carbohydrate chemistry, it can be employed for modifying and functionalizing carbohydrates, including the introduction of azide groups. These azide-modified carbohydrates can participate in click chemistry reactions, enabling the development of bioconjugates and glycoarrays. In drug discovery, this compound aids in the design and synthesis of carbohydrate-based therapeutics, such as glycosidase inhibitors or glycoconjugate vaccines, by modifying carbohydrate structures. For biochemical research, this compound is valuable in studying carbohydrate-protein interactions, glycan biosynthesis, and glycoprotein engineering. It allows for the modification and labeling of carbohydrates for subsequent biological assays and analyses. Finally, as an azide derivative, it finds application in click chemistry reactions, specifically the azide-alkyne cycloaddition reaction (commonly known as the "click reaction"). This reaction enables efficient and selective labeling, bioconjugation, and cross-linking in various biological and materials science applications.
특징 및 장점
β-D-Glucopyranosyl azide, 2-(acetylamino)-2-deoxy-, 3,4,6-triacetate is a complex carbohydrate and the glycosylation product of 2,3,4,6-tetraacetyl α--D--glucose and 2,3,6 -tri--O--acetyl--2--deoxy--β--D--glucopyranose. This compound has been modified by Click Chemistry with 4-(dimethylamino)pyridine (DMAP). The modification has produced an acetamido group at the C2 position of the glucopyranoside moiety. The compound is available in high purity for research purposes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Brendan L Wilkinson et al.
Journal of medicinal chemistry, 51(6), 1945-1953 (2008-03-01)
A library of glycoconjugate benzene sulfonamides have been synthesized and investigated for their ability to inhibit the enzymatic activity of physiologically relevant human carbonic anhydrase (hCA) isozymes: hCA I, II, and tumor-associated IX. Our synthetic strategy directly links the known
Synthesis and Biological Evaluation of Novel Carbohydrate-Derived Derivatives of Erlotinib
Yu, Wenbo
Drug Development Research, 77, 319-325 (2016)
Georg C Rudolf et al.
Chembiochem : a European journal of chemical biology, 14(18), 2447-2455 (2013-10-30)
Copper-catalysed alkyne-azide 1,3-dipolar cycloaddition (CuAAC) is the predominantly used bioconjugation method in the field of activity-based protein profiling (ABPP). Several limitations, however, including conversion efficiency, protein denaturation and buffer compatibility, restrict the scope of established procedures. We introduce an ABPP
Wenbo Yu et al.
Drug development research, 77(6), 319-325 (2016-08-16)
Preclinical Research A series of novel carbohydrate-derived Erlotinib derivatives were prepared by the copper-catalyzed cycloaddition reaction of erlotinib with various azido-sugars. The structures of the newly synthesized compounds were characterized and their cytostatic effects evaluated in vitro on human cancer
Copper-assisted click reactions for activity-based proteomics: fine-tuned ligands and refined conditions extend the scope of application
Rudolf, Georg et. al.
Chembiochem, 14, 2447-2455 (2013)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.