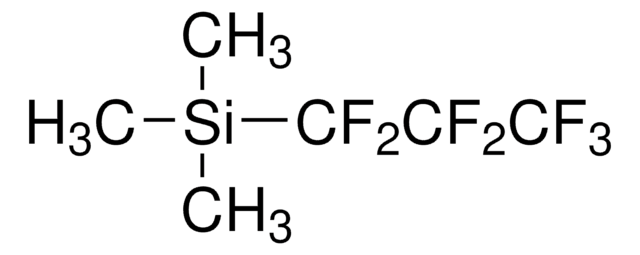모든 사진(1)
About This Item
실험식(Hill 표기법):
C5H9F5Si
CAS Number:
Molecular Weight:
192.20
MDL number:
UNSPSC 코드:
12352101
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
97%
형태
liquid
refractive index
n/D 1.325
density
1.095 g/mL
SMILES string
C[Si](C)(C)C(F)(F)C(F)(F)F
InChI
1S/C5H9F5Si/c1-11(2,3)5(9,10)4(6,7)8/h1-3H3
InChI key
MTPVUVINMAGMJL-UHFFFAOYSA-N
일반 설명
Trimethylpentafluoroethylsilane ((Pentafluoroethyl)trimethylsilane) is a perfluoroalkylsilane. Alkyl triflates undergo nucleophilic pentafluoroethylation with trimethylpentafluoroethylsilane to form the corresponding pentafluoroethylated alkanes.
애플리케이션
Trimethylpentafluoroethylsilane ((Pentafluoroethyl)trimethylsilane) may be used as a perfluoroalkylating reagent for the synthesis of the following quinoline derivatives:
- 5-bromo-2-(perfluoroethyl)quinoline
- 8-methoxy-2-(perfluoroethyl)quinoline
- 1-(perfluoroethyl)isoquinoline
- 8-(tert-butoxy)-5,7-dichloro-2-(perfluoroethyl)quinolone
Trimethylpentafluoroethylsilane has been reported as a Ruppert-Prakash type reagent for the addition of pentafluoroethane. Recent report by Larionov and coworkers displayed the feasibility of adding pentrafluoroethane to N-heterocycles under basic conditions.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
1.4 °F - closed cup - (calculated)
Flash Point (°C)
-17 °C - closed cup - (calculated)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
A Facile New Method for the Two-step Substitution of Hydroxy Groups in Primary Alcohols for Trifluoromethyl and Pentafluoroethyl Moieties.
Sevenard DV, et al.
Synlett, 2001(03), 0379-0381 (2001)
Experimental determination of the conformational free energies (A values) of fluorinated substituents in cyclohexane by dynamic 19 F NMR spectroscopy. Part 2. Extension to fluoromethyl, difluoromethyl, pentafluoroethyl, trifluoromethylthio and trifluoromethoxy groups.
Carcenac Y, et al.
New. J. Chem., 30(3), 447-457 (2006)
David E Stephens et al.
Organic & biomolecular chemistry, 12(32), 6190-6199 (2014-07-06)
The scope and mechanistic implications of the direct transformation of heterocyclic N-oxides to 2-trifluoromethyl-, and related perfluoroalkyl- and perfluoroaryl-substituted N-heterocycles has been studied. The reaction is effected by perfluoroalkyl- and perfluorophenyltrimethylsilane in the presence of strong base. In situ displacement
관련 콘텐츠
The major research interests of Prof. Jinbo Hu's lab include the development of new fluorination reagents and reactions, especially the difluoromethylation, difluoromethylenation, and monofluoromethylation methods.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.