665533
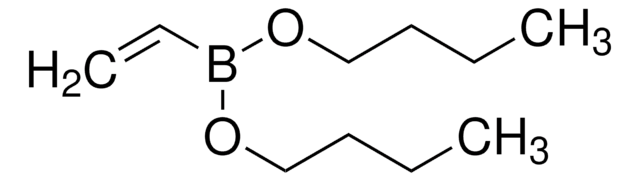
trans-2-(3-Chlorophenyl)vinylboronic acid pinacol ester
97%
동의어(들):
2-(E-2-(3-Chlorophenyl)vinyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C14H18BClO2
CAS Number:
Molecular Weight:
264.56
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
97%
refractive index
n20/D 1.536
bp
138-140 °C/0.9-1.0 mmHg
density
1.049 g/mL at 25 °C
SMILES string
CC1(C)OB(OC1(C)C)\C=C\c2cccc(Cl)c2
InChI
1S/C14H18BClO2/c1-13(2)14(3,4)18-15(17-13)9-8-11-6-5-7-12(16)10-11/h5-10H,1-4H3/b9-8+
InChI key
NZBKTAJNGYXYSQ-CMDGGOBGSA-N
애플리케이션
trans-2-(3-Chlorophenyl)vinylboronic acid pinacol ester can be used as a reactant:
- To prepare aryl derivatives by C−C bond formation via palladium-catalyzed Suzuki−Miyaura reaction.
- In the diastereoselective synthesis of alkenes via K3PO4-promoted transition metal-free nucleophilic substitution of unactivated alkyl triflates.
- To synthesize (3-chlorophenyl)cyclopropyl boronic acid pinacol ester by reacting with diazomethane.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
Mild Base Promoted Nucleophilic Substitution of Unactivated sp3-Carbon Electrophiles with Alkenylboronic Acids
Liu Shiwen, et al.
advanced synthesis and catalysis, 360(19), 3667-3671 (2018)
Automated library synthesis of cyclopropyl boronic esters employing diazomethane in a tube-in-tube flow reactor
Koolman HF, et al.
Organic & Biomolecular Chemistry, 14(27), 6591-6595 (2016)
Mild Base Promoted Nucleophilic Substitution of Unactivated sp3-Carbon Electrophiles with Alkenylboronic Acids
Liu Shiwen, et al.
Advanced Synthesis & Catalysis, 360(19), 3667-3671 (2018)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.