추천 제품
분석
97%
mp
43-48 °C (lit.)
저장 온도
2-8°C
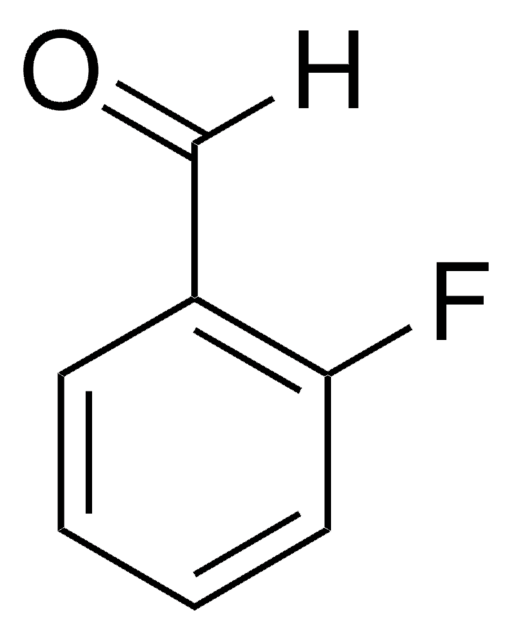
SMILES string
COc1ccc(C=O)c(F)c1
InChI
1S/C8H7FO2/c1-11-7-3-2-6(5-10)8(9)4-7/h2-5H,1H3
InChI key
UNWQNFJBBWXFBG-UHFFFAOYSA-N
일반 설명
2-Fluoro-4-methoxybenzaldehyde is a fluorinated aromatic aldehyde. It can be prepared from 4-bromo-3-fluoroanisole.
애플리케이션
2-Fluoro-4-methoxybenzaldehyde may be used in the preparation of:
- fluorine containing 2,4,5-trisubstituted imidazole
- 1-(2-fluoro-4-methoxyphenyl)-2-propanone
- 6-(2-fluoro-4-methoxyphenyl)fulvene
- 10-(2-fluoro-4-methoxyphenyl)-6,7,9,10-tetrahydro-1Hfuro[3,4-b]pyrazolo[3,4-f]quinolin-9-one
- polyhydroquinoline (PHQ)
- 3-(2-fluoro-4-methoxyphenyl) acrylic acid methyl ester
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Indium trifluoride: A highly efficient catalyst for the synthesis of fluorine-containing 2, 4, 5-trisubstituted imidazoles under solvent-free conditions.
Reddy MV and Jeong YT.
Journal of Fluorine Chemistry, 142, 45-51 (2012)
Fluorinated derivatives of titanocene Y: synthesis and cytotoxicity studies.
Claffey J, et al.
European Journal of Organic Chemistry, 26, 4074-4082 (2008)
Synthesis, cytotoxic activity and docking studies of new 4-aza-podophyllotoxin derivatives.
Hatti I, et al.
Medicinal Chemistry Research, 24(8), 3305-3313 (2015)
Synthesis of 1, 3-Bis (hydroxy-halogenophenyl)-propane-1, 3-diamines and their Pt (II) Complexes, Syntheses of the Ligands.
Kammermeier T and Wiegrebe W.
Arch. Pharm. (Weinheim), 327, 547-561 (1994)
Jack G Parsons et al.
Molecules (Basel, Switzerland), 9(6), 449-458 (2007-11-17)
The synthesis of (2S)-2-benzyloxymethyl-3-(2-fluoro-4-methoxyphenyl)- propionic acid, (2S)-2-benzyloxymethyl-3-(2-fluoro-4-methylphenyl)propionic acid and (2S)-2-benzyl-oxymethyl-3-(2,4-dimethylphenyl)propionic acid has been achieved by TiCl4 mediated alkylation of the corresponding (4R)-4-benzyl-3-[3-(2-fluoro-4-methoxyphenyl-, 2-fluoro-4-methylphenyl-, 2,4- dimethylphenyl-)propionyl]-2-oxazolidinones, followed by hydrolysis of the chiral auxiliary. The stereochemistry of the alkylation reaction was confirmed
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.