추천 제품
분석
99%
mp
114-116 °C (lit.)
SMILES string
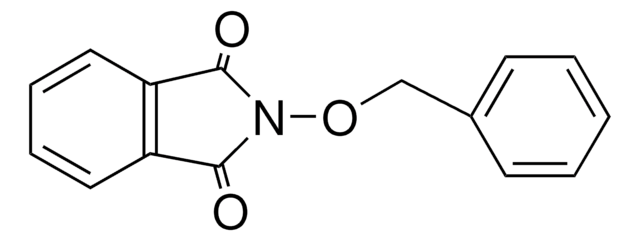
O=C1N(Cc2ccccc2)C(=O)c3ccccc13
InChI
1S/C15H11NO2/c17-14-12-8-4-5-9-13(12)15(18)16(14)10-11-6-2-1-3-7-11/h1-9H,10H2
InChI key
WITXFYCLPDFRNM-UHFFFAOYSA-N
일반 설명
N-Benzylphthalimide (NBPT), also known as 2-benzylisoindoline-1,3-dione, is an N-substituted phthalimide. It has been prepared by reacting phthalic anhydride with benzyl amine in glacial acetic acid. Vibrational spectra of NBPT has been recorded and assigned. NBPT is a roof-shaped molecule with a planar cyclic imide and a phenyl ring connected by a methylene group. Crystal structure of N-benzylphthalimide has parallel layers of phthalimides stack along the a axis.
애플리케이션
N-Benzylphthalimide may be used in the following syntheses:
- 2-benzyl-1,1,3,3-tetraphenylisoindoline
- tailor-made highly fluorous porphyrin derivatives
- N-benzylisoindole
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
The direct conversion of phthalimides to isoindoles.
Garmaise DL and Ryan A.
Journal of Heterocyclic Chemistry, 7(2), 413-413 (1970)
Impact of molecular size on electron spin relaxation rates of nitroxyl radicals in glassy solvents between 100 and 300 K.
Sato HIDEO, et al.
Molecular Physics, 105(15-16), 2137-2151 (2007)
Vibrational assignment of N-benzylphthalimide and 15N-benzylphthalimide.
Kolev T and Juchnovski I.
Journal of Molecular Structure, 349, 377-380 (1995)
N-benzylphthalimide.
Warzecha K-D, et al.
Acta Crystallographica Section E, Structure Reports Online, 62(6), 2367-2368 (2006)
Highly fluorous porphyrins as model compounds for molecule interferometry.
Tuxen J, et al.
European Journal of Organic Chemistry, 25, 4823-4833 (2011)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.