모든 사진(1)
About This Item
실험식(Hill 표기법):
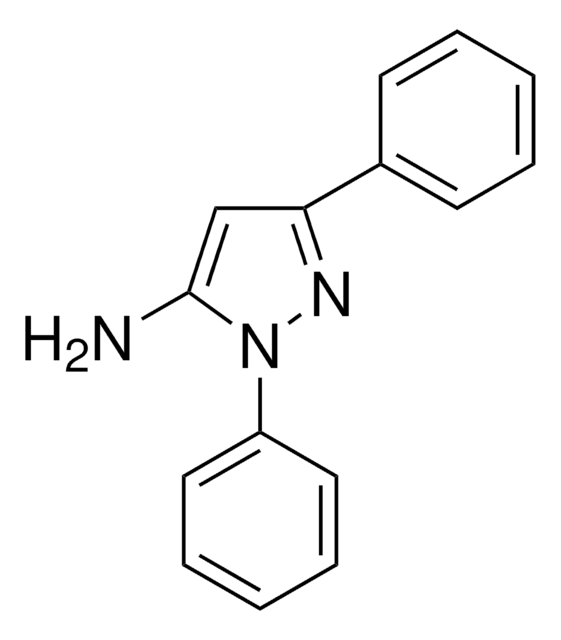
C9H9N3
CAS Number:
Molecular Weight:
159.19
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
98%
mp
124-127 °C (lit.)
SMILES string
Nc1cc([nH]n1)-c2ccccc2
InChI
1S/C9H9N3/c10-9-6-8(11-12-9)7-4-2-1-3-5-7/h1-6H,(H3,10,11,12)
InChI key
PWSZRRFDVPMZGM-UHFFFAOYSA-N
일반 설명
3-Amino-5-phenylpyrazole (3-phenyl-1H-pyrazol-5-amine), an amino pyrazole derivative, is an aza-heterocyclic amine. It has been reported to be synthesized by heating either 3-amino-4-bromo- or 3-amino-5-phenylisothiazole in the presence of anhydrous hydrazine. On reaction with ZnCl2 it affords chlorido-tris(3-amino-5-phenyl-1Hpyrazole-N2)zinc (II) chloride.
애플리케이션
3-Amino-5-phenylpyrazole ((3-phenyl-1H-pyrazol-5-amine) may be used in the synthesis of the following:
- Urea derivatives by reaction with azido(6-(benzofuran-2-yl)-2-methylpyridin-3-yl) methanone.
- 2-Mercaptoacetamide analogs by treating with thioglycolic acid.
- 3-(Substituentpyrimidayl)-5,6-benzocoumarins by treating with 3-(2′-formyl-1′-chlorovinyl)-5,6-benzocoumarin.
- Substituted 2,7-diphenylpyrazolo[1,5-a]pyrimidine-5-carboxylic esters by reacting with substituted β-diketo esters.
- N-ethoxycarbonylthiourea derivative by reacting with ethoxycarbonyl isothiocyanate.
- Heterobiaryl pyrazolo[3,4-b]pyridines by reacting with indole-3-carboxaldehyde.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Crystal structure of chlorido-tris (3-amino-5-phenyl-1H pyrazole-N2) zinc (II) chloride, [ZnCl (C9H9N3)3] Cl.
Jacimovic ZK, et al
Zeitschrift fur Kristallographie, 226(3), 397-399 (2011)
Scott T Moe et al.
Bioorganic & medicinal chemistry, 17(8), 3072-3079 (2009-03-31)
Botulinum neurotoxin elicits its paralytic activity through a zinc-dependant metalloprotease that cleaves proteins involved in neurotransmitter release. Currently, no drugs are available to reverse the effects of botulinum intoxication. Herein we report the design of a novel series of mercaptoacetamide
Recent advances in the chemistry of ethoxycarbonyl isothiocyanate and related compounds.
George B and Papadopoulos EP
Journal of Heterocyclic Chemistry, 20(5), 1127-1142 (1983)
Convenient synthesis of some new pyrazolo [5, 1-c] triazines, isoxazolo [3, 4-d] pyrimidine and pyridine derivatives containing benzofuran moiety.
Abdelhamid AO, et al
European Journal of Chemistry, 3(2), 129-137 (2012)
Syntheses of 3-pyrimidyl-and 3-pyranyl-5, 6-benzocoumarin derivatives.
El-Deen IM, et al
Bull. Korean Chem. Soc., 23(4), 610-612 (2002)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.