추천 제품
분석
97%
형태
powder
반응 적합성
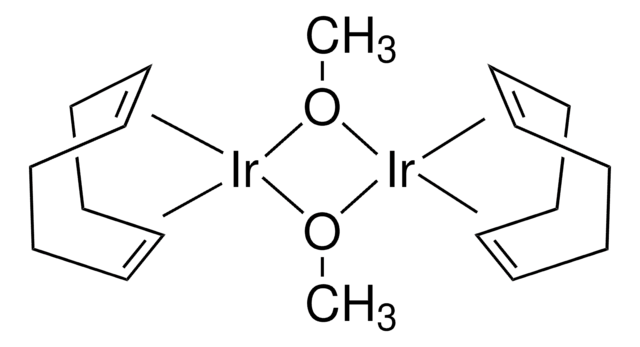
core: iridium
reagent type: catalyst
mp
160-165 °C (dec.) (lit.)
SMILES string
Cl[Ir].Cl[Ir].[CH]1[CH]CCCCCC1.[CH]2[CH]CCCCCC2.[CH]3[CH]CCCCCC3.[CH]4[CH]CCCCCC4
InChI
1S/4C8H14.2ClH.2Ir/c4*1-2-4-6-8-7-5-3-1;;;;/h4*1-2H,3-8H2;2*1H;;/q;;;;;;2*+1/p-2/b4*2-1-;;;;
InChI key
WBRREXQCZAFSKS-XFCUKONHSA-L
애플리케이션
- Isomerization-hydroboration reactions with nido-carboranyldiphosphine as stabilizing ligand
- Hydrogen peroxide oxidation of hydroxamic acids and their subsequent hetero Diels-Alder cycloaddition reactions
- Immobilization of organic functional groups onto solid supports through vinylsilane coupling reactions
- Alkylation reactions
- Guerbet reaction
- Allylic amination reactions in a DNA-diene-iridium(I) hybrid system
- Asymmetric hydroamination reactions
- Isomerization-hydroboration reactions with nido-carboranyldiphosphine as stabilizing ligand.
- Hydrogen peroxide oxidation of hydroxamic acids and their subsequent hetero Diels-Alder cycloaddition reactions.
- Immobilization of organic functional groups onto solid supports through vinylsilane coupling reactions.
- Alkylation reactions.
- Guerbet reaction.
- Allylic amination reactions using a DNA-diene-iridium(I) hybrid system.
- Asymmetric hydroamination reactions.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
문서
Arylboronic acids and esters, vital tools in chemical transformations, find extensive use, particularly in the Suzuki-Miyaura cross-coupling reaction.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.








![[Ir(cod)(acac)] Umicore](/deepweb/assets/sigmaaldrich/product/structures/188/615/470bfca9-6b61-476a-9486-f7da61962e4c/640/470bfca9-6b61-476a-9486-f7da61962e4c.png)