추천 제품
분석
97%
형태
powder
mp
184-186 °C (lit.)
형광
λex 341 nm; λem 376 nm in methanol
λex 342 nm; λem 395 nm (Reaction product)
SMILES string
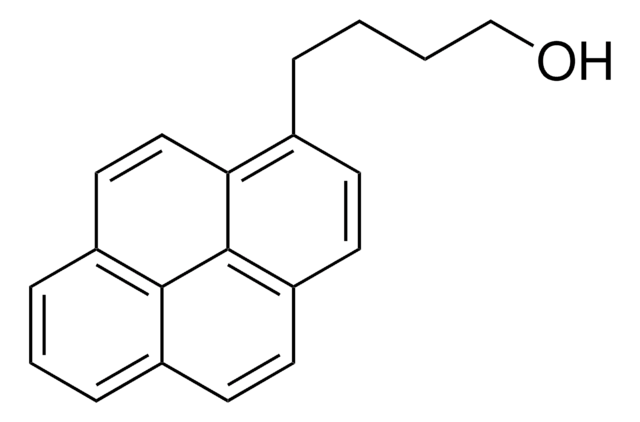
OC(=O)CCCc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C20H16O2/c21-18(22)6-2-3-13-7-8-16-10-9-14-4-1-5-15-11-12-17(13)20(16)19(14)15/h1,4-5,7-12H,2-3,6H2,(H,21,22)
InChI key
QXYRRCOJHNZVDJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
1-Pyrenebutyric acid (PBA) is a conjugated polymer that has a large π system and a carboxylic group. It is majorly used in surface functionalization. It has a high fluorescence efficiency and stability that make it useful in optoelectronic applications.
애플리케이션
PBA can be grafted on the surface of graphene and can be used in sensors. It can also be in the fabrication of ultracapacitor electrodes as alternative storage systems. It can also be used in the formation of multi-layered film for photovoltaic applications.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
On monolayer formation of pyrenebutyric acid on graphene
Hinnemo M, et al.
Langmuir, 33(15), 3588-3593 (2017)
Yongzheng Yang et al.
Organic & biomolecular chemistry, 4(9), 1746-1754 (2006-04-25)
FRET-based fluorogenic substrates for lipases and esterases were prepared in four steps from commercially available building blocks. The substrates are pyrenebutyric acid monoesters of aliphatic 1,2-diols bearing a dinitrophenylamino group as a quencher. The most enzyme-reactive substrate is ester 2a.
Wei Song et al.
Biosensors & bioelectronics, 26(7), 3181-3186 (2011-01-25)
A highly efficient enzyme-based screen printed electrode (SPE) was obtained by using covalent attachment between 1-pyrenebutanoic acid, succinimidyl ester (PASE) adsorbing on the graphene oxide (GO) sheets and amines of tyrosinase-protected gold nanoparticles (Tyr-Au). Herein, the bi-functional molecule PASE was
Ming-Yuan Wei et al.
Biosensors & bioelectronics, 24(9), 2909-2914 (2009-03-27)
A redox-labeled direct competitive electrochemical immunoassay for polycyclic aromatic hydrocarbons (PAHs) was developed. A ruthenium tris(bipyridine)-pyrenebutyric acid conjugate was synthesized as the redox-labeled tracer. Its recognition by an anti-PAH monoclonal antibody was confirmed by surface plasmon resonance. In the immunoassay
Peter Guterstam et al.
Biochimica et biophysica acta, 1788(12), 2509-2517 (2009-10-03)
Cell-penetrating peptides (CPPs) are membrane permeable vectors recognized for their intrinsic ability to gain access to the cell interior. The hydrophobic counter-anion, pyrenebutyrate, enhances cellular uptake of oligoarginine CPPs. To elucidate CPP uptake mechanisms, the effect of pyrenebutyrate on well-recognized
문서
Nitric oxide (NO) as a signal transporter in neurons, endothelial cells and in the immune system.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.