모든 사진(2)
About This Item
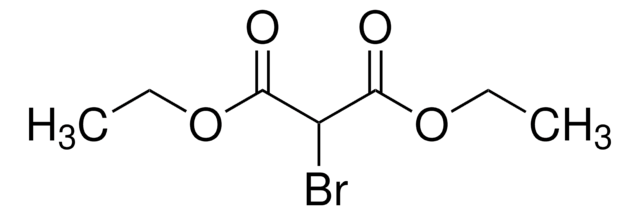
실험식(Hill 표기법):
C7H16S2Si
CAS Number:
Molecular Weight:
192.42
Beilstein:
1616463
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
≥99%
refractive index
n20/D 1.533 (lit.)
bp
54-55 °C/0.17 mmHg (lit.)
density
1.014 g/mL at 25 °C (lit.)
작용기
thioether
SMILES string
C[Si](C)(C)C1SCCCS1
InChI
1S/C7H16S2Si/c1-10(2,3)7-8-5-4-6-9-7/h7H,4-6H2,1-3H3
InChI key
BTTUMVHWIAXYPJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
2-(Trimethylsilyl)-1,3-dithiane participates in Lewis base-catalyzed 1,3-dithiane addition to electrophiles such as carbonyl compounds and N-substituted aldimines. It undergoes novel diazo transfer reaction with tosyl azide in hexamethylphosphoramide-THF to yield 2-diazo-1,3-dithiane, which on decomposition yields formal carbene adducts. It is a versatile acyl anion equivalent.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
204.8 °F - closed cup
Flash Point (°C)
96 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Diazo transfer reaction of 2-(trimethylsilyl)-1, 3-dithiane with tosyl azide. Carbenic reactivity of transient 2-diazo-1, 3-dithiane.
Benati L, et al.
Tetrahedron, 53(27), 9269-9278 (1997)
Makoto Michida et al.
Chemistry, an Asian journal, 3(8-9), 1592-1600 (2008-06-21)
Lewis base-catalyzed 1,3-dithiane addition to electrophiles such as carbonyl compounds and N-substituted aldimines with 2-trimethylsilyl-1,3-dithiane (TMS-dithiane) is described. By the activation of the carbon-silicon bond in the presence of a Lewis base catalyst such as tetrabutylammonium phenoxide (PhONnBu(4)), a 1,3-dithiane
Smith, A. B., III; Boldi, A. M.
Journal of the American Chemical Society, 119, 6925-6925 (1997)
Amos B Smith et al.
Journal of the American Chemical Society, 125(47), 14435-14445 (2003-11-20)
The development, application, and advantages of a one-flask multicomponent dithiane linchpin coupling protocol, over the more conventional stepwise addition of dithiane anions to electrophiles leading to the rapid, efficient, and stereocontrolled assembly of highly functionalized intermediates for complex molecule synthesis
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.