추천 제품
분석
98%
mp
147-149 °C (lit.)
SMILES string
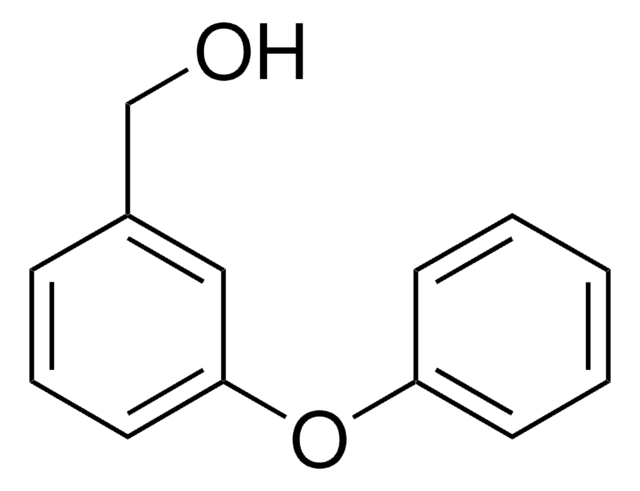
OC(=O)c1cccc(Oc2ccccc2)c1
InChI
1S/C13H10O3/c14-13(15)10-5-4-8-12(9-10)16-11-6-2-1-3-7-11/h1-9H,(H,14,15)
InChI key
NXTDJHZGHOFSQG-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
3-Phenoxybenzoic acid was used as standard in the determination of urinary residues of 3-phenoxybenzoic acid by GLC with electron-capture detection method. It was also used in the synthesis of poly(ether-ketones).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Improved syntheses of poly (oxy-1, 3-phenylenecarbonyl-1, 4-phenylene) and related poly (ether-ketones) using polyphosphoric acid/P 2 O 5 as polymerization medium.
Baek J-B and Tan L-S.
Polymer, 44(15), 4135-4147 (2003)
C Aprea et al.
Journal of chromatography. B, Biomedical sciences and applications, 695(2), 227-236 (1997-08-01)
The determination of urinary 3-phenoxybenzoic acid enables exposure to pyrethroid insecticides to be evaluated. A method for the quantitative determination of this metabolite in urine is described. The compound and the internal standard (2-phenoxybenzoic acid) are derivatized with pentafluorobenzylbromide and
Idalina Bragança et al.
Environmental science and pollution research international, 26(3), 2987-2997 (2018-12-07)
3-Phenoxybenzoic acid (3-PBA) is a shared metabolite of several synthetic pyrethroid pesticides (SPs) resulting from environmental degradation of parent compounds and thus occurs frequently as a residue in samples. Hence, the importance of 3-PBA evaluation after pyrethroid application. There is
Grafting of vapor-grown carbon nanofibers via in-situ polycondensation of 3-phenoxybenzoic acid in poly (phosphoric acid).
Baek J-B, et al.
Macromolecules, 37(22), 8278-8285 (2004)
Kateryna Babina et al.
Environment international, 48, 109-120 (2012-08-16)
Organophosphorus (OP) and pyrethroid (PYR) compounds are the most widely used insecticides. OPs and PYRs are developmental neurotoxicants. Understanding the extent of exposure in the general population and especially in young children is important for the development of public health
문서
Friedel-Crafts acylation with Lewis acid catalysts forms monoacylated products via electrophilic aromatic substitution of arenes.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.