추천 제품
분석
97%
형태
solid
mp
101-103 °C (lit.)
작용기
nitro
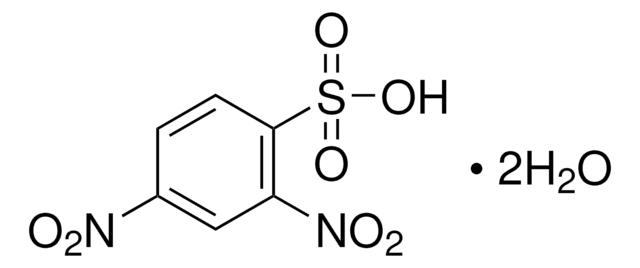
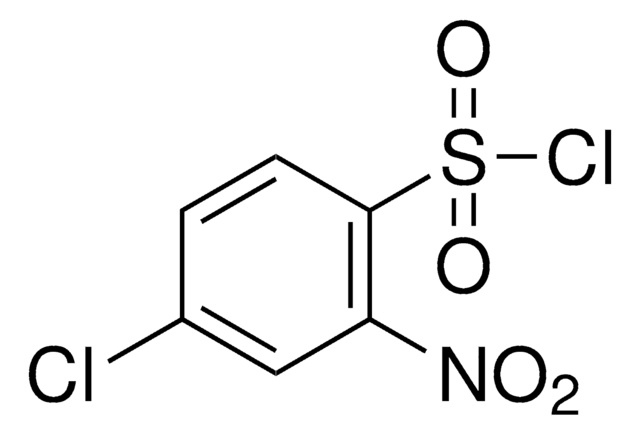
SMILES string
[O-][N+](=O)c1ccc(c(c1)[N+]([O-])=O)S(Cl)(=O)=O
InChI
1S/C6H3ClN2O6S/c7-16(14,15)6-2-1-4(8(10)11)3-5(6)9(12)13/h1-3H
InChI key
SSFSNKZUKDBPIT-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
2,4-Dinitrobenzenesulfonyl chloride causes the sulfonation of glycosylamines to yield N-glycosyl-2,4-dinitrobenzenesulfonamides.
May contain up to 3.5% benzene
애플리케이션
2,4-Dinitrobenzenesulfonyl chloride was used to protect primary amines. It was used as starting reagent in the synthesis of tert-butyl 2-[(2,4-dinitrophenyl) sulfonyl]aminoacetate.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Carc. 1A - Eye Dam. 1 - Muta. 1B - Skin Corr. 1B - STOT RE 2
표적 기관
Blood
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Rachel J Ball et al.
Artificial DNA, PNA & XNA, 1(1), 27-35 (2011-06-21)
Halogen-labelled peptide organic acid (HPOA) monomers have been synthesised and incorporated into sequence-specific peptide nucleic acid (PNA) probes. Three different types of probe have been prepared; the unmodified PNA probe, the PNA probe with a mass marker, and the PNA
Vishwanath Gaitonde et al.
Journal of carbohydrate chemistry, 31(4-6), 353-370 (2013-01-26)
The N-glycosyl-2,4-dinitrobenzenesulfonamides were accessed via benzoyl-protected β-glycosyl azides. The azides were reduced with Adams' catalyst to the corresponding amines. The glycosylamines were sulfonated with 2,4-dinitrobenzenesulfonyl chloride to form N-glycosyl-2,4-dinitrobenzenesulfonamides in moderate yields. β-Glycosyl amides were then prepared in 67 -
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.