추천 제품
분석
97%
형태
solid
refractive index
n20/D 1.564 (lit.)
bp
102-104 °C/2 mmHg (lit.)
density
1.09 g/mL at 25 °C (lit.)
SMILES string
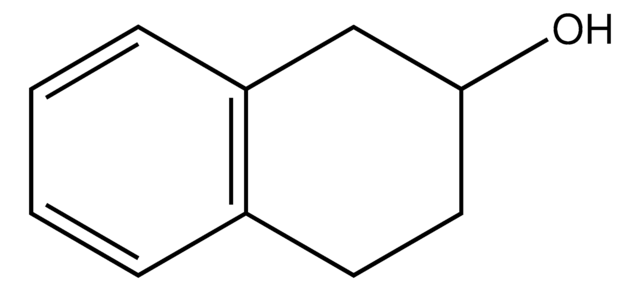
OC1CCCc2ccccc12
InChI
1S/C10H12O/c11-10-7-3-5-8-4-1-2-6-9(8)10/h1-2,4,6,10-11H,3,5,7H2
InChI key
JAAJQSRLGAYGKZ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
(R)-(-)-enantiomer of 1,2,3,4-Tetrahydro-1-naphthol is a substrate for aryl sulfotransferase (AST) IV enzyme and (S)-(+)-1,2,3,4-tetrahydro-1-naphthol is a competitive inhibitor of AST IV-catalyzed sulfation of 1-naphthalenemethanol. It is the major urinary metabolite of tetralin.
애플리케이션
1,2,3,4-Tetrahydro-1-naphthol was used as chiral probe to examine the role of three aromatic residues in enzyme-substrate interactions at the sulfuryl acceptor binding site of aryl sulfotransferase IV enzyme.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Metabolism of tetralin and toxicity of Cuprex in man.
D E Drayer et al.
Drug metabolism and disposition: the biological fate of chemicals, 1(3), 577-579 (1973-05-01)
Jonathan J Sheng et al.
Drug metabolism and disposition: the biological fate of chemicals, 32(5), 559-565 (2004-04-22)
Aryl sulfotransferase (AST) IV (also named tyrosine-ester sulfotransferase and ST1A1) is a major phenol sulfotransferase in the rat, and it catalyzes the sulfation of many drugs, carcinogens, and other xenobiotics that contain phenol, benzylic alcohol, N-hydroxy arylamine, and oxime functional
Vyas Sharma et al.
Journal of medicinal chemistry, 45(25), 5514-5522 (2002-12-03)
Comparative Molecular Field Analysis (CoMFA) methods were used to produce a 3D-QSAR model that correlated the catalytic efficiency of rat hepatic aryl sulfotransferase (AST) IV, expressed as log(k(cat)/K(m)), with the molecular structures of its substrates. A total of 35 substrate
W F Leebaw et al.
The Journal of clinical endocrinology and metabolism, 47(3), 480-487 (1978-09-01)
Although the role of the neurotransmitter, dopamine (DA), in the regulation of PRL has been well documented, controversy exists regarding its participation in the regulation of the other pituitary hormones. Consequently, we infused DA into six healthy male subjects (ages
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.