UHPLC Analysis of Riboflavin and Impurities on Titan C18 with MS Detection
Materials
standard
CONDITIONS
column
Titan C18, 10 cm x 2.1 mm I.D., 1.9 μm particles (577124-U)
mobile phase
[A] 0.1% formic acid in water; [B] 0.1% formic acid in acetonitrile (95:5)
gradient
5% to 25% in 6 min, return to 5% b in 0.1 min, and re-equilibrate for 4 min
flow rate
500 μL/min
pressure
5000 psi (345 bar)
column temp.
35 °C
detector
ESI (+), MRM and TIC
detector
Diode Array at 276 nm
injection
2 μL
Description
Analysis Note
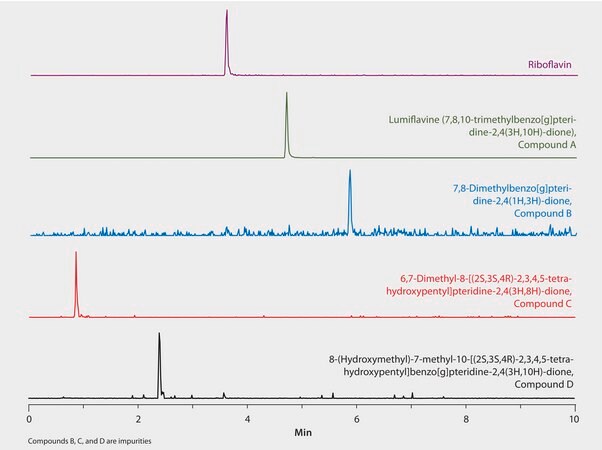
Riboflavin (vitamin B2) is a water-soluble vitamin. It is synthesized by all plants and many microorganisms, but it is not produced by higher animals. It is a precursor of coenzymes that are required for the enzymatic oxidation of carbohydrates, so it is essential to basic metabolism. Riboflavin is used as an additive to food and feed and is also used in fortification of baby food and cereal. The current USP method for impurity analysis is non-quantitative, and uses an ion pair reagent in the mobile phase. An alternative to USP and EP methods using LC-MS/MS to identify and quantify the riboflavin and impurities is presented. This new proposed method is also compatible with UV-Vis detection.
A riboflavin certified reference material (CRM) was used in this study. MRM transitions for the determination of ions for the parent and four impurites were made. Three transitions were used for each ion. Structures for each of these major impurities were confirmed by MS. In addition to improving the specificity of the method with MS/MS detection, two additional goals were to decrease analysis time and eliminate the need for ion pairing reagents that are not compatible with MS.
Other Notes
Sample:
USP and EP methods use 0.1 M NaOH to solubilize riboflavin.
Dilute with mobile phase as necessary.
Legal Information
null