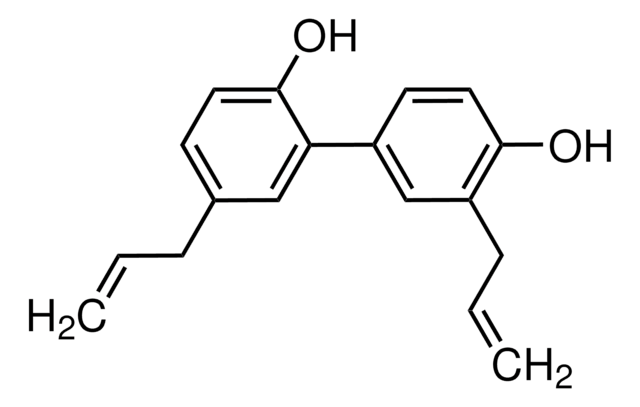
G8761
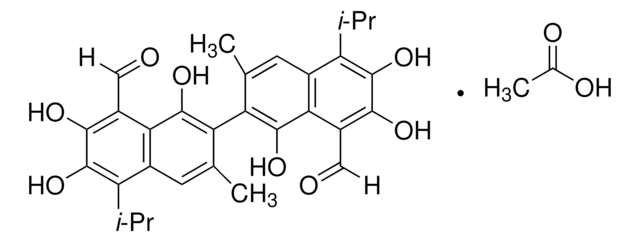
(±)-Gossypol from cotton seeds
≥95% (HPLC), powder, PAF antagonist/inhibitor
Synonym(s):
(±)-2,2′-bis(8-Formyl-1,6,7-trihydroxy-5-isopropyl-3-methylnaphthalene)
About This Item
Recommended Products
product name
(±)-Gossypol from cotton seeds, ≥95% (HPLC)
Quality Level
Assay
≥95% (HPLC)
storage temp.
2-8°C
SMILES string
O=C([H])C1=C(C(O)=C(C2=C(C)C=C(C(C(C)C)=C(O)C(O)=C3C([H])=O)C3=C2O)C(C)=C4)C4=C(C(C)C)C(O)=C1O
InChI
1S/C30H30O8/c1-11(2)19-15-7-13(5)21(27(35)23(15)17(9-31)25(33)29(19)37)22-14(6)8-16-20(12(3)4)30(38)26(34)18(10-32)24(16)28(22)36/h7-12,33-38H,1-6H3
InChI key
QBKSWRVVCFFDOT-UHFFFAOYSA-N
Gene Information
human ... AKR1B1(231) , BAD(572) , BCL2(596) , LDHA(3939) , LDHB(3945) , MCL1(4170)
General description
Application
- as a standard to quantify the gossypol content in the extracts of leaves and flower buds
- to study its antifertility effects on spermatogenesis in NMRI mice
- to analyse its impact on human spermatozoa
- to incorporate into the artificial diet for larval rearing
Biochem/physiol Actions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Warburg effect enhances glucose to lactate conversion in tumor cells, regardless of oxygen levels; impacting cancer metabolism since 1924.
Warburg effect enhances glucose to lactate conversion in tumor cells, regardless of oxygen levels; impacting cancer metabolism since 1924.
Warburg effect enhances glucose to lactate conversion in tumor cells, regardless of oxygen levels; impacting cancer metabolism since 1924.
Warburg effect enhances glucose to lactate conversion in tumor cells, regardless of oxygen levels; impacting cancer metabolism since 1924.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service