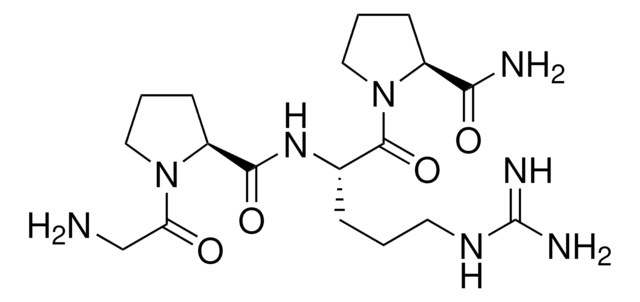
G4541
Gly-Gly-His
≥98% (TLC)
Synonym(s):
Copper-binding peptide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H15N5O4
CAS Number:
Molecular Weight:
269.26
MDL number:
UNSPSC Code:
12352209
NACRES:
NA.26
Recommended Products
Product Name
Gly-Gly-His,
Assay
≥98% (TLC)
Quality Level
form
solid
color
white
storage temp.
−20°C
InChI
1S/C10H15N5O4/c11-2-8(16)13-4-9(17)15-7(10(18)19)1-6-3-12-5-14-6/h3,5,7H,1-2,4,11H2,(H,12,14)(H,13,16)(H,15,17)(H,18,19)
InChI key
PDAWDNVHMUKWJR-UHFFFAOYSA-N
Related Categories
Amino Acid Sequence
Gly-Gly-His
General description
Gly-Gly-His (GGH) tripeptide with carboxyl-terminal histidine residue is hydrophilic.
Application
Gly-Gly-His has been used:
- as a peptide test probe in the hydrophilic interaction liquid chromatography (HILIC) studies
- in the electrochemical modification of back-side contact transducers (BSC) electrodes in copper (Cu2+) sensing studies
- for the fabrication carbon nanotube electrode for detection of Cu2+ ions
Biochem/physiol Actions
Gly-Gly-His (GGH) tripeptide displays the affinity of copper (Cu2+) complexation. These complexes display good skin tolerance and may be useful in wound healing. GGH copper complexes are proposed to reduce tumor necrosis factor α (TNF-α)-based interleukin 6 (IL-6) secretion by in vivo studies with fibroblasts. GGH is used in comparative studies with other imidazole-containing peptides such as carnosine. An area of active application research involves the GGH binding of copper ions.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A P Dicks et al.
Chemistry & biology, 3(8), 655-659 (1996-08-01)
We have recently shown that S-nitrosothiols (RSNOs) decompose in aqueous buffer to give nitric oxide, an important signalling molecule, and the corresponding disulphides. This occurs by reaction with Cu+ generated from Cu2+ (supplied as hydrated Cu2+) by thiolate reduction. To
Meng Lin et al.
Biosensors & bioelectronics, 26(2), 940-945 (2010-07-16)
We developed a novel biosensor comprising a transducer of carboxyl end-capped overoxidized polypyrrole nanowire electrode and a probe of tripeptide (Gly-Gly-His) selectively cognitive of Cu2+. The developed sensor was demonstrated to specifically detect Cu2+ in the nanomolar range. The diameter
R W Hay et al.
Journal of inorganic biochemistry, 52(1), 17-25 (1993-10-01)
The protonation constants of the tripeptide glycylglycyl-L-histidine (L-) have been determined at 25 degrees C and I = 0.1 mol dm-3 as log K 8.06, 6.82, and 2.80. Complexation with copper(II) can be represented by the series of equilibria [formula:
Meng Lin et al.
Analytica chimica acta, 746, 63-69 (2012-09-15)
A novel electrochemical biosensor based on functionalized polypyrrole (PPy) nanotube arrays modified with a tripeptide (Gly-Gly-His) proved to be highly effective for electrochemical analysis of copper ions (Cu(2+)). The vertically oriented PPy nanotube arrays were electropolymerized by using modified zinc
Wen Jiang et al.
Journal of chromatography. A, 1127(1-2), 82-91 (2006-07-04)
A novel phosphorylcholine type zwitterionic stationary phase was synthesized by graft polymerization of 2-methacryloyloxyethyl phosphorylcholine onto the surface of porous silica particles. The resulting material possesses both negatively charged phosphoric acid and positively charged quaternary ammonium groups, which renders it
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service