C3175
Chloramphenicol-Water Soluble
powder, suitable for cell culture, BioReagent
Synonym(s):
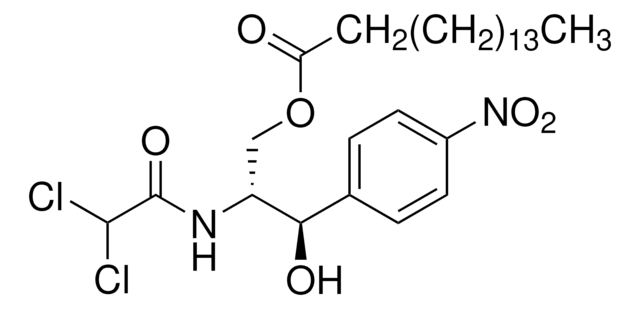
Chloramphenicol, Chlornitromycin, Chlorocid, Chloromycetin, Cloramfenicol, D-(−)-threo-2-Dichloroacetamido-1-(4-nitrophenyl)-1,3-propanediol, D-(−)-threo-2,2-Dichloro-N-[β-hydroxy-α-(hydroxymethyl)-β-(4-nitrophenyl)ethyl]acetamide, Chloromycetin, D-threo-2,2-Dichloro-N-[β-hydroxy-α-(hydroxymethyl)-4-nitrophenethyl]aceta, Kloramfenikol
About This Item
Recommended Products
product name
Chloramphenicol-Water Soluble, powder, BioReagent, suitable for cell culture
product line
BioReagent
Quality Level
form
powder
technique(s)
cell culture | mammalian: suitable
solubility
H2O: 50-500 mg/mL (stock solution)
PBS: 50-500 mg/mL (stock solution)
other salt solutions: not recommended
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mycobacteria
mycoplasma
Mode of action
protein synthesis | interferes
Application
- as a supplement in Luria-Bertani (LB) medium to culture Escherichia coli
- as a supplement in LB agar to culture Citrobacter rodentium
- to culture Toxoplasma gondii
Biochem/physiol Actions
Packaging
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Antibiotic kill curve is a dose response experiment in which mammalian cells are subjected to increasing amounts of selection antibiotic
Antibiotic kill curve is a dose response experiment in which mammalian cells are subjected to increasing amounts of selection antibiotic
Antibiotic kill curve is a dose response experiment in which mammalian cells are subjected to increasing amounts of selection antibiotic
Antibiotic kill curve is a dose response experiment in which mammalian cells are subjected to increasing amounts of selection antibiotic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service