92243
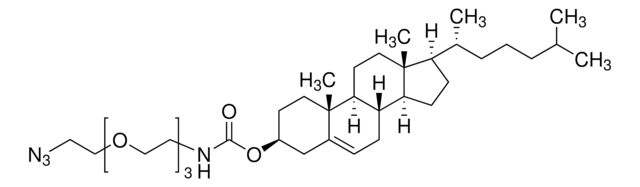
Cholesteryl N-(2-dimethylaminoethyl)carbamate
≥98% (TLC)
Synonym(s):
3β-{N-[2-(Dimethylamino)ethyl]carbamoyl}cholesterol, DC-Chol
About This Item
Recommended Products
Assay
≥98% (TLC)
form
powder or crystals
functional group
ester
storage temp.
−20°C
SMILES string
CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4CC(CC[C@]4(C)[C@H]3CC[C@]12C)OC(=O)NCCN(C)C
InChI
1S/C32H56N2O2/c1-22(2)9-8-10-23(3)27-13-14-28-26-12-11-24-21-25(36-30(35)33-19-20-34(6)7)15-17-31(24,4)29(26)16-18-32(27,28)5/h11,22-23,25-29H,8-10,12-21H2,1-7H3,(H,33,35)/t23-,25?,26+,27-,28+,29+,31+,32-/m1/s1
InChI key
HIHOWBSBBDRPDW-MTIZRNOUSA-N
Related Categories
Application
- Role of interleukin-6 in antigen-specific mucosal immunoglobulin A induction by cationic liposomes.: This study investigates the use of cholesteryl N-(2-dimethylaminoethyl)carbamate in cationic liposomes for enhancing mucosal immunoglobulin A production, highlighting its potential in vaccine development (Tada et al., 2021).
- Nasal vaccination with pneumococcal surface protein A in combination with cationic liposomes consisting of DOTAP and DC-chol confers antigen-mediated protective immunity against Streptococcus pneumoniae infections in mice.: This research demonstrates the efficacy of cholesteryl N-(2-dimethylaminoethyl)carbamate in nasal vaccines, providing a promising strategy for respiratory infection prevention (Tada et al., 2018).
Biochem/physiol Actions
Packaging
Hazard Statements
Hazard Classifications
Aquatic Chronic 4
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service