131105
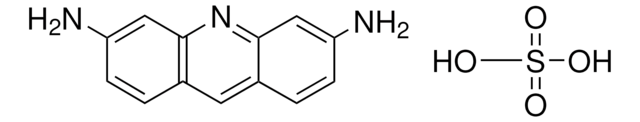
3,6-Diaminoacridine hydrochloride
Dye content 95 %
Synonym(s):
Proflavine hydrochloride, Proflavine monohydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C13H11N3 · HCl
CAS Number:
Molecular Weight:
245.71
EC Number:
MDL number:
UNSPSC Code:
12171500
PubChem Substance ID:
NACRES:
NA.47
Recommended Products
Assay
94.0% (HPLC)
form
powder
composition
Dye content, 95%
mp
270 °C (dec.) (lit.)
solubility
H2O: 1 mg/mL, clear
λmax
456 nm
ε (extinction coefficient)
50000 at 260 nm in methanol
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
Cl[H].Nc1ccc2cc3ccc(N)cc3nc2c1
InChI
1S/C13H11N3.ClH/c14-10-3-1-8-5-9-2-4-11(15)7-13(9)16-12(8)6-10;/h1-7H,14-15H2;1H
InChI key
PBBGTVBGXBUVLT-UHFFFAOYSA-N
Application
3, 6-Diaminoacridine hydrochloride has been used for studying cation transfer at the liquid–liquid interface.
An intercalating dye.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anirban Basu et al.
Journal of photochemistry and photobiology. B, Biology, 165, 42-50 (2016-10-22)
Interaction of proflavine with hemoglobin (Hgb) was studied employing spectroscopy, calorimetry, and atomic force microscopy. The equilibrium constant was found to be of the order 10
Anna Porfireva et al.
Nanomaterials (Basel, Switzerland), 10(5) (2020-05-14)
A DNA sensor has been developed for the determination of doxorubicin by consecutive electropolymerization of an equimolar mixture of Azure B and proflavine and adsorption of native DNA from salmon sperm on a polymer film. Electrochemical investigation showed a difference
Anirban Basu et al.
Journal of biomolecular structure & dynamics, 38(6), 1590-1597 (2019-05-07)
The binding of proflavine, an acriflavine derivative, with the RNA polynucletodide polyadenylic acid-polyuridylic acid is investigated here to understand the structural and thermodynamic basis of the binding process. Such binding data are crucial for designing viable theraperutic agents. Spectroscopic studies
Miklós Nagy et al.
Scientific reports, 9(1), 8250-8250 (2019-06-05)
Amino-isocyanoacridines (ICAAcs), as first members of their class, turned out to be a novel, multifunctional acridine orange (AO) type dye family with a number of additional favorable properties. They have enhanced solvatochromic emission range, low quantum yields (ΦF = 2.9-0.4%) in water
Membrane activity of ionisable drugs ? a task for liquid?liquid electrochemistry?
Malkia A
Electrochemical Communications, 5:6, 473-479 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service