M79204
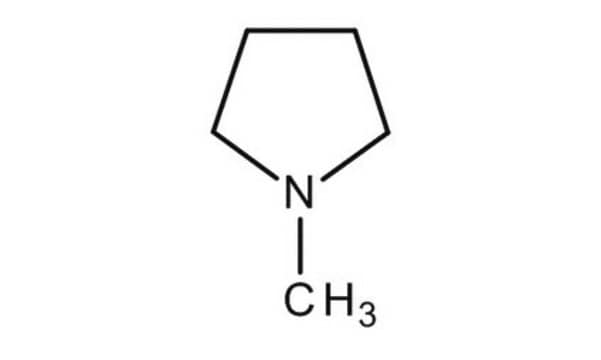
N-Methylpyrrolidine
97%
Synonym(s):
1-Methylpyrrolidine, N-Methylpyrrolidine, N-Methyltetrahydropyrrole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H11N
CAS Number:
Molecular Weight:
85.15
Beilstein:
102445
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
bp
80-81 °C (lit.)
density
0.819 g/mL at 25 °C (lit.)
SMILES string
CN1CCCC1
InChI
1S/C5H11N/c1-6-4-2-3-5-6/h2-5H2,1H3
InChI key
AVFZOVWCLRSYKC-UHFFFAOYSA-N
Related Categories
Application
N-Methylpyrrolidine can be used as a starting material to synthesize:
N-methyl pyrrolidine-zinc borohydride (ZBHNMP), a reducing agent for reduction of aldehydes, ketones, acid chlorides, and esters. ZBHNMP is also used in the reductive amination of aldehydes and ketones to their corresponding amines.
N-alkyl-N-methyl-pyrrolidinium bis(trifluoromethanesulfonyl)imide ionic liquids.
N-methylpyrrolidine-2-one hydrotribromide applicable as a catalyst for aziridination of alkenes.
N-methyl pyrrolidine-zinc borohydride (ZBHNMP), a reducing agent for reduction of aldehydes, ketones, acid chlorides, and esters. ZBHNMP is also used in the reductive amination of aldehydes and ketones to their corresponding amines.
N-alkyl-N-methyl-pyrrolidinium bis(trifluoromethanesulfonyl)imide ionic liquids.
N-methylpyrrolidine-2-one hydrotribromide applicable as a catalyst for aziridination of alkenes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Aquatic Chronic 2 - Flam. Liq. 2 - Skin Corr. 1A
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-0.4 °F - closed cup
Flash Point(C)
-18 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
N-methylpyrrolidine-2-one hydrotribromide: An efficient and new catalyst for the aziridination of alkenes using Chloramine-T under solvent free conditions
Jain SL, et al.
J. Mol. Catal. A: Chem., 256(1-2), 16-20 (2006)
Reductive amination of aldehydes and ketones to their corresponding amines with N-methylpyrrolidine zinc borohydride
Alinezhad H, et al.
Tetrahedron Letters, 50(6), 659-661 (2009)
Effect of the alkyl group on the synthesis and the electrochemical properties of N-alkyl-N-methyl-pyrrolidinium bis (trifluoromethanesulfonyl) imide ionic liquids
Appetecchi GB, et al.
Electrochimica Acta, 54(4), 1325-1332 (2009)
Inhibition of acetylcholinesterase by caffeine, anabasine, methyl pyrrolidine and their derivatives.
N Karadsheh et al.
Toxicology letters, 55(3), 335-342 (1991-03-01)
The inhibition of acetylcholinesterase (AChE) by caffeine, anabasine, methylpyrrolidine and several derivatives was examined. Most of the compounds had moderate inhibitory activity with I50 values in the range of 87-480 microM. The inhibition of AChE by these compounds has not
N-Methylpyrrolidine-zinc borohydride: As a new stable and efficient reducing agent in organic synthesis
Tajbakhsh M, et al.
Synthetic Communications, 33(2), 229-236 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service