675725
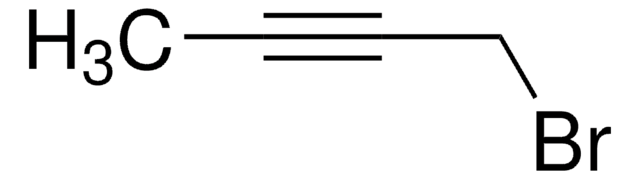
4-Bromo-1-butyne
97%
Synonym(s):
1-Bromo-3-butyne
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H5Br
CAS Number:
Molecular Weight:
132.99
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.481
density
1.417 g/mL at 25 °C
SMILES string
BrCCC#C
InChI
1S/C4H5Br/c1-2-3-4-5/h1H,3-4H2
InChI key
XLYOGWXIKVUXCL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Bromo-1-butyne is commonly used as a reactant. It serves as a source of alkyl halides for the introduction of bromo functionality into the molecule.
Application
4-Bromo-1-butyne is used as a reactant in the synthesis of:
- Macrocycles by cobalt-mediated [2+2+2] co-cyclotrimerization.
- 2,4,5-trisubstituted oxazoles by a gold-catalyzed formal [3+2] cycloaddition.
- Intramolecular 1,3-dipolar cycloaddition to synthesize 1,3,4-oxadiazoles.
- Lactones bearing alkynes for reductive cyclization in the preparation of azulene derivatives.
- Substituted α-pyrones by gold-catalyzed coupling reactions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Flam. Liq. 3 - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
75.0 °F
Flash Point(C)
23.9 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Intermolecular and Selective Synthesis of 2, 4, 5-Trisubstituted Oxazoles by a Gold-Catalyzed Formal [3+ 2] Cycloaddition.
Davies PW, et al.
Angewandte Chemie (International Edition in English), 50(38), 8931-8935 (2011)
Reductive Cyclization Cascades of Lactones Using SmI2- H2O.
Parmar D, et al.
Journal of the American Chemical Society, 133(8), 2418-2420 (2011)
Synthesis of macrocycles via cobalt-mediated [2+ 2+ 2] cycloadditions.
Bonaga LVR, et al.
Journal of the American Chemical Society, 127(10), 3473-3485 (2005)
A facile synthesis of N-C linked 1, 2, 3-triazole-oligomers
V Fiandanese, et al.
Tetrahedron, 67, 5254-5260 (2011)
Intramolecular Diels- Alder/1, 3-dipolar cycloaddition cascade of 1, 3, 4-oxadiazoles.
Elliott GI, et al.
Journal of the American Chemical Society, 128(32), 10589-10595 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service