457701
(S)-(−)-2-Methyl-CBS-oxazaborolidine solution
1 M in toluene
Synonym(s):
α,α-Diphenyl-L-prolinol methylboronic acid cycl-amide ester, (S)-1-Methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2c][1,3,2]oxazaborole, (S)-3,3-Diphenyl-1-methyltetrahydro-3H-pyrrolo[1,2-c][1,3,2]oxazaborole, (S)-Tetrahydro-1-methyl-3,3-diphenyl-1H,3H-pyrrolo[1,2-c][1,3,2]oxazaborole
About This Item
Recommended Products
Quality Level
concentration
1 M in toluene
bp
111 °C
density
0.929 g/mL at 25 °C
storage temp.
room temp
SMILES string
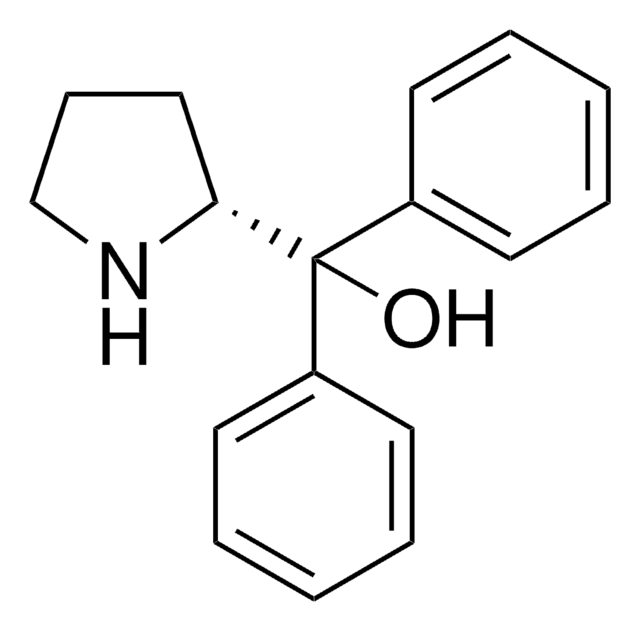
CB1OC([C@@H]2CCCN12)(c3ccccc3)c4ccccc4
InChI
1S/C18H20BNO/c1-19-20-14-8-13-17(20)18(21-19,15-9-4-2-5-10-15)16-11-6-3-7-12-16/h2-7,9-12,17H,8,13-14H2,1H3/t17-/m0/s1
InChI key
VMKAFJQFKBASMU-KRWDZBQOSA-N
Looking for similar products? Visit Product Comparison Guide
Application
It may also be used in the preparation of:
- (1S)-2-azido-1-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)ethanol
- (R)-α-deuteriobenzyl alcohol
- (R)-2-(1-hydroxyethyl)benzo[b]thiophene
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 2 - STOT SE 3
Target Organs
Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
39.2 °F - closed cup
Flash Point(C)
4 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
we are pleased to offer both enatiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1M solution in toluene.
Learn about the applications of chiral oxazaborolidinium ions (COBIs) as Lewis acid catalysts in different asymmetric reactions such as cyclopropanation, epoxidation, and radical reactions along with details of their catalytic action.
Learn about the applications of chiral oxazaborolidinium ions (COBIs) as Lewis acid catalysts in different asymmetric reactions such as cyclopropanation, epoxidation, and radical reactions along with details of their catalytic action.
Learn about the applications of chiral oxazaborolidinium ions (COBIs) as Lewis acid catalysts in different asymmetric reactions such as cyclopropanation, epoxidation, and radical reactions along with details of their catalytic action.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![(S,S)-(+)-2,6-Bis[2-(hydroxydiphenylmethyl)-1-pyrrolidinyl-methyl]-4-methylphenol 95%](/deepweb/assets/sigmaaldrich/product/structures/126/939/bff1a61c-8335-434f-8da4-9b1929aef17f/640/bff1a61c-8335-434f-8da4-9b1929aef17f.png)




