160660
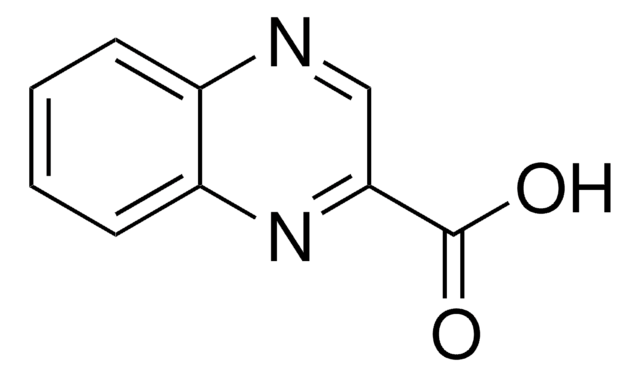
Quinaldic acid
98%
Synonym(s):
2-Quinolinecarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H7NO2
CAS Number:
Molecular Weight:
173.17
Beilstein:
126322
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
156-158 °C (lit.)
SMILES string
OC(=O)c1ccc2ccccc2n1
InChI
1S/C10H7NO2/c12-10(13)9-6-5-7-3-1-2-4-8(7)11-9/h1-6H,(H,12,13)
InChI key
LOAUVZALPPNFOQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Quinaldic acid is also referred as quinoline-2-carboxylic acid. Microwave-assisted preparation of substituted anilides of quinaldic acid has been reported. It inhibits the oxidation of pyruvate, α-ketoglutarate, glutamate and citrate in rat liver mitochondria. Quinaldic acid is a metabolite of tryptophan degradation and inhibits the gluconeogenesis in perfused livers.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Alleyn T Plowright et al.
Chemistry & biology, 9(5), 607-618 (2002-05-29)
Saframycin A (SafA) is a natural product that inhibits human cancer cell proliferation. Its synthetic analog, QAD, is a more potent inhibitor of these cells. SafA does not affect wild-type yeast, but it does inhibit growth of the strain CCY333
Induction of ornithine decarboxylase activity in mouse urinary bladder by L-tryptophan and some of its metabolites.
M Matsushima et al.
Cancer research, 42(9), 3587-3591 (1982-09-01)
Graham Smith et al.
Acta crystallographica. Section C, Crystal structure communications, 64(Pt 3), o180-o183 (2008-03-07)
The structures of the 1:1 proton-transfer compounds of 4,5-dichlorophthalic acid with 8-hydroxyquinoline, 8-aminoquinoline and quinoline-2-carboxylic acid (quinaldic acid), namely anhydrous 8-hydroxyquinolinium 2-carboxy-4,5-dichlorobenzoate, C(9)H(8)NO(+) x C(8)H(3)Cl(2)O(4)(-), (I), 8-aminoquinolinium 2-carboxy-4,5-dichlorobenzoate, C(9)H(9)N(2)(+) x C(8)H(3)Cl(2)O(4)(-), (II), and the adduct hydrate 2-carboxyquinolinium 2-carboxy-4,5-dichlorobenzoate quinolinium-2-carboxylate monohydrate
Paloma Riquelme et al.
Nature communications, 9(1), 2858-2858 (2018-07-22)
Human regulatory macrophages (Mreg) have shown early clinical promise as a cell-based adjunct immunosuppressive therapy in solid organ transplantation. It is hypothesised that recipient CD4+ T cell responses are actively regulated through direct allorecognition of donor-derived Mregs. Here we show
Zhao-Qing Du et al.
Journal of Cancer, 11(15), 4614-4624 (2020-06-04)
Platelet-derived growth receptor α (PDGFRα) is a key factor in many pathophysiological processes. The expression level of PDGFRα is significantly elevated in the early stage of liver development and maintained at a lower level in adult normal livers. In this
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service