103039
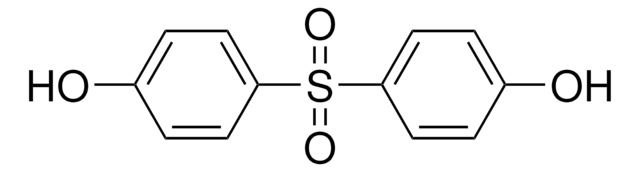
4,4′-Sulfonyldiphenol
98%
Synonym(s):
4-Hydroxyphenyl sulfone, Bis(4-hydroxyphenyl) sulfone, Bisphenol S
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
O2S(C6H4OH)2
CAS Number:
Molecular Weight:
250.27
Beilstein:
2052954
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
form
solid
mp
245-250 °C (lit.)
SMILES string
Oc1ccc(cc1)S(=O)(=O)c2ccc(O)cc2
InChI
1S/C12H10O4S/c13-9-1-5-11(6-2-9)17(15,16)12-7-3-10(14)4-8-12/h1-8,13-14H
InChI key
VPWNQTHUCYMVMZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4,4′-Sulfonyldiphenol also known as Bisphenol S(BPS) is a monomer characterized by its sulfone group (-SO2-) linking two phenolic rings. Its unique chemical structure imparts high thermal stability, chemical resistance, and tensile strength. It is often used as a monomer or additive in the synthesis of high-performance polymers, particularly polyethersulfones (PES) and polyphenylsulfones (PPSU). It is widely used in the field of polymer synthesis, medical devices, and dental composites.
Application
4,4′-Sulfonyldiphenol can be used:
- As a monomer to synthesize fibrous poly(arylene ether ketone)s with excellent water affinity, thermal stability and film forming capability. This can be utilized to fabricate Proton exchange membranes for electrodialysis, fuel cells and flow batteries.
- As a precursor to prepare polyphosphazene-based contrast agents for magnetic resonance imaging (MRI) and computed tomography (CT). It enhances the thermal stability, chemical resistance and allows easy functionalization of contrast agents.
- As a monomer to prepare poly (cyclotriphosphazene-co-4,4-sulfonyldiphenol) (PZS) coating for separators, in lithium-ion battery. The coated separators show sufficient enhancement of liquid electrolyte wettability and ionic conductivity, resulting in better cycle performance.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Erica Danzl et al.
International journal of environmental research and public health, 6(4), 1472-1484 (2009-05-15)
A group of compounds structurally similar to bis(4-hydroxyphenyl)propane (bisphenol A, BPA) are called bisphenols (BPs), and some of them can partially replace BPA in industrial applications. The production and consumption of BPs other than BPA, especially those of bis(4-hydroxyphenyl)methane (bisphenol
Elise Grignard et al.
Toxicology in vitro : an international journal published in association with BIBRA, 26(5), 727-731 (2012-04-18)
In 2011, the European Commission has restricted the use of Bisphenol A in plastic infant feeding bottles. In a response to this restriction, Bisphenol S is now often used as a component of plastic substitutes for the production of babybottles.
Imèn Khmiri et al.
Environment international, 138, 105644-105644 (2020-03-18)
The measurement of bisphenol-S (BPS) and its glucurono-conjugate (BPSG) in urine may be used for the biomonitoring of exposure in populations. However, this requires a thorough knowledge of their toxicokinetics. The time courses of BPS and BPSG were assessed in
René Viñas et al.
Environmental health perspectives, 121(3), 352-358 (2013-03-06)
Bisphenol A (BPA) is a well-known endocrine disruptor that imperfectly mimics the effects of physiologic estrogens via membrane-bound estrogen receptors (mERα, mERβ, and GPER/GPR30), thereby initiating nongenomic signaling. Bisphenol S (BPS) is an alternative to BPA in plastic consumer products
José-Manuel Molina-Molina et al.
Toxicology and applied pharmacology, 272(1), 127-136 (2013-05-30)
Bisphenols are a group of chemicals structurally similar to bisphenol-A (BPA) in current use as the primary raw material in the production of polycarbonate and epoxy resins. Some bisphenols are intended to replace BPA in several industrial applications. This is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service