86320
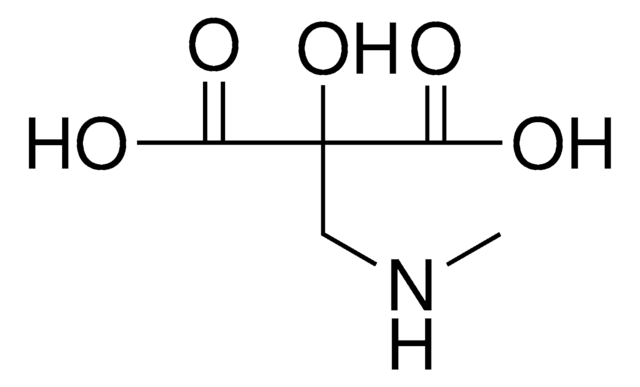
Tartronic acid
≥97.0%
Synonym(s):
Hydroxymalonic acid, Hydroxypropanedioic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOCH(COOH)2
CAS Number:
Molecular Weight:
120.06
Beilstein:
1209791
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0%
form
powder
mp
158-160 °C (dec.) (lit.)
storage temp.
2-8°C
SMILES string
OC(C(O)=O)C(O)=O
InChI
1S/C3H4O5/c4-1(2(5)6)3(7)8/h1,4H,(H,5,6)(H,7,8)
InChI key
ROBFUDYVXSDBQM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Polymer synthesis for enhanced thermal conductivity: Tartronic acid is used to exploit enzyme reactions in polymer synthesis, significantly increasing the thermal conductivity of materials, which is pivotal in manufacturing and material science applications (Nan et al., 2023).
- Advances in green chemical treatments: This acid plays a role in the electro-oxidation pathways for treating glycerol waste, contributing to sustainable chemical processes and green chemistry applications, which are essential for reducing environmental impact (Cheng et al., 2021).
- Development in biodiesel by-products treatment: Tartronic acid is also involved in kinetic studies for the electrochemical conversion of glycerol, a by-product of biodiesel production, highlighting its role in renewable energy and waste valorization (Pérès et al., 2020).
- Base-free oxidation reactions: It aids in the development of base-free conditions for glycerol to glyceraldehyde oxidation reactions over platinum-based catalysts, offering advancements in catalysis and organic synthesis processes (Capron et al., 2019).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G Gutierrez et al.
Journal of applied physiology (Bethesda, Md. : 1985), 76(6), 2735-2741 (1994-06-01)
Lactate uptake by skeletal muscle occurs under diverse conditions, including hypoxia and electrical stimulation. A possible metabolic fate of lactate in resting muscle is its conversion to pyruvate followed by carboxylation to malate in the cytosolic malic reaction. To test
S H Park et al.
Biochemistry, 23(23), 5446-5453 (1984-11-06)
Measurement of the initial rate of the malic enzyme reaction varying the concentration of NAD at several different fixed levels of Mg2+ (0.25-1.0 mM) and a single malate concentration gave a pattern which intersects to the left of the ordinate.
A Shah et al.
Bioorganic & medicinal chemistry, 5(2), 323-334 (1997-02-01)
A new, aromatic analogue of the EPSP synthase enzyme reaction intermediate 1 has been identified, which contains a 3-hydroxymalonate moiety in place of the usual 3-phosphate group. This simplified inhibitor was readily prepared in five steps from ethyl 3,4-dihydroxybenzoate. The
J Klimek et al.
Journal of steroid biochemistry, 26(1), 161-163 (1987-01-01)
It has been shown that the conversion of cholesterol to progesterone by human term placental mitochondria incubated in the presence of malate or fumarate was inhibited by hydroxymalonate--an inhibitor of malic enzyme. No inhibition was observed when mitochondria were incubated
Yolande A Chan et al.
Biochemistry, 49(17), 3667-3677 (2010-04-01)
Polyketide synthases elongate a polyketide backbone by condensing carboxylic acid precursors that are thioesterified to either coenzyme A or an acyl carrier protein (ACP). Two of the three known ACP-linked extender units, (2S)-aminomalonyl-ACP and (2R)-hydroxymalonyl-ACP, are found in the biosynthesis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service